New family of proteins linked to major role in cancer
(Medical Xpress)—Scientists have described a new family of proteins that appear to play a key role in cancer and might be targets for future cancer drugs. A major new study in the journal Nature sets out the structure of the new family, called glutamate intramembrane proteases – the founding member of which plays a critical role in transforming healthy cells into cancer cells.
The research, funded by Cancer Research UK and conducted by scientists at The Institute of Cancer Research, London, defined the structure of a protein called Rce1, and established it as the first known member of a whole new protein family.
The research was conducted on Methanococcus maripaludis Rce1, a homologue of human Rce1. It has relevance to cancer in humans because Rce1 helps control another class of proteins (the CAAX proteins) involved in cell division and the transformation into cancer.
These CAAX proteins include one of the most important of all triggers for cancer – the Ras protein – which is enormously important for turning cells cancerous but has been difficult to target with traditional drugs.
Understanding the other proteins that are required for Ras to trigger cancer is therefore enormously important, since they may prove easier targets for precision drug treatment.
The researchers chose M. Maripaludis Rce1 from a shortlist of around 30 versions from different organisms including humans, yeast and bacteria. They selected the M. Maripaludis version as the most suitable for the crystallisation process needed to study the protein in detail. Although M. Maripaludis Rce1 is quite different from human Rce1, it shares important elements of its structure.
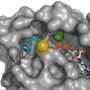
Using a second bacterium, Escherichia coli, as a protein factory, the researchers manufactured M. Maripaludis Rce1 before purifying and then crystallising it. Using a technique called X-ray diffraction, they were able to examine the structure of Rce1 in unprecedented detail.
They found that Rce1 had a completely different structure to any other known family of transmembrane proteins, and a different sequence of amino acids, the building blocks which make up proteins.
Transmembrane proteins stretch from inside to the outside of membranes to pass on messages from one side to the other and govern many of the cellular processes in all forms of life. Rce1 is an intramembrane protease, which means it can snip off particular parts of other membrane-associated proteins.
In the study, extensive experiments were unable to put Rce1 within any of the three known families of intramembrane proteases, leaving the researchers to conclude they had found a fourth, the glutamate intramembrane proteases, of which Rce1 is the only known member.
Professor David Barford, who led the study as Professor of Molecular Biology at The Institute of Cancer Research, London, before taking up a new position at the Medical Research Council Laboratory of Molecular Biology in Cambridge, said:
"Against our expectations, we found the Rce1 protein is so different from any other protein known to science that we need to put it in its own family. Previous studies have found Rce1 interacts with the Ras pathway, which plays a key role in many different types of cancer, so establishing the protein's unique structure is an important step forward.
"Our study could help lead to new potential cancer treatments that target the Ras signalling pathway, but that possibility is still a way off. Our findings underline just how much of the fundamental processes of life we still do not understand, and could give other cancer researchers their first step on a possible route to new treatments."
Professor Alan Ashworth, Chief Executive of The Institute of Cancer Research, London, said:
"It's a rare and important moment when scientists discover a new class of proteins, and it is exciting not only for the new insight it gives us, but also for the potential it creates for new anti-cancer strategies."
Dr Sarah Hazell, senior science information officer at Cancer Research UK, said:
"Ras is an important molecule in the development of many cancers, so research that adds to our understanding of how this molecule is controlled is very important. We look forward to the next step of this research – seeing how it might be used to develop new ways of stopping Ras in its tracks in patients."
More information: Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1, DOI: 10.1038/nature12754