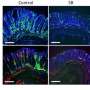
Gut microorganisms may determine cancer treatment outcome
An intact population of microorganisms that derive food and benefit from other organisms living in the intestine is required for optimal response to cancer therapy, according to a mouse study by scientists at the National Cancer Institute (NCI), part of the National Institutes of Health, and their collaborators.
NCI scientists found that tumors of germ-free mice (mice completely lacking these microorganisms), or mice treated with antibiotics to deplete the gut of bacteria, were largely impaired in their ability to respond to immunotherapy that slows cancer growth and prolongs survival. The mice were also impaired in their ability to respond to mainstay chemotherapy drugs such as oxaliplatin and cisplatin. These findings in mice may underscore the importance of microorganisms in optimal cancer treatment outcomes in humans. The study, led by Romina Goldszmid, Ph.D., staff scientist, NCI, and Giorgio Trinchieri, M.D., director of the Cancer and Inflammation Program, Center for Cancer Research, NCI, appeared Nov. 22, 2013, in Science.
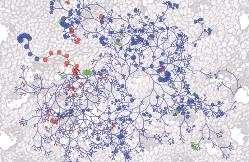
Image of a Evolutionary tree of mouse gut bacteria superimposed over an image of colon tissue with larger circles indicating greater abundance of bacteria. Red circles are bacteria that prime mice to respond to immunotherapy; green shows bacteria that suppress anti-tumor response to the drug.
Gut commensal microbiota are microorganisms that live in the gut and thrive but do not affect their host, in this case laboratory mice. Humans also harbor gut commensal microbiota that can influence local and body-wide inflammation as well as modify the tumor microenvironment, which consists of cells, signaling molecules and mechanisms that may support tumor growth and also cause drug resistance.
To study the importance of commensal bacteria, the scientists used mice raised in sterile conditions from birth so they did not harbor any bacteria, or alternatively, conventionally raised mice that received a potent antibiotic cocktail that is known to decrease bacteria by more than 10,000–fold. The mice received these antibiotics in their drinking water, starting three weeks prior to tumor inoculation. T hey continued to receive doses of the antibiotic cocktail throughout the experiment.
To analyze tumors at comparable stages of progression, lymphoma, colon, and melanoma cancers that could be transplanted were selected, based on their susceptibility to therapeutic drugs. Cancer cells from these tumors were then injected under the skin of the mice, where they formed tumors that grew to reach a diameter of one-fifth of an inch or more. The tumors were then treated with an immunotherapy that included CpG-oligonucleotides, which stimulated the immune system, or with the chemotherapy drugs oxaliplatin and cisplatin, which attacked the tumors.
Germ-free mice, or mice that received the antibiotic cocktail, responded poorly to drug therapy for their tumors. This resulted in a lower production of cytokines (proteins secreted by lymph cells that affects cellular activity and controls inflammation) as well as lower tumor death therefore demonstrating that optimal responses to cancer therapy required an intact commensal microbiota.
In an independent co-submitted study that will appear in the same issue of Science, Laurence Zitvogel, M.D., Ph.D., Gustave Roussy Institute, Paris, and colleagues showed that a different type of chemotherapy drug, cyclophosphamide, altered the composition of the intestinal microbiota and damaged the intestinal wall, thereby affecting optimal anti-tumor immune response and the therapeutic effectiveness of cyclophosphamide.
"The use of antibiotics should be considered as an important element affecting microbiota composition. It has been demonstrated, and our present study has confirmed, that after antibiotic treatment the bacterial composition in the gut never returns to its initial composition," said Trinchieri. "Thus, our findings raise the possibility that the frequent use of antibiotics during a patient's lifetime or to treat infections related to cancer and its side-effects may affect the success of anti-cancer therapy."
In next steps, Goldszmid and Trinchieri will work in mice to fully characterize the molecular signaling by which the bacteria in the gut can actually send messages to distant organs or tumors and change the type and level of inflammation present in those organs. They also plan to characterize, in humans, the role of gut bacteria on the bodies' inflammatory response and tumor response to therapy. Additionally, the researchers plan to design clinical studies by giving antibiotics to healthy volunteers to study their effect on the molecular mechanisms regulating inflammation.
More information: "Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment," by N. Iida et al. Science, 2013.