Study reveals evolution at work: Analyses elucidate a part of the brain particular to primates
New research by UC Santa Barbara's Kenneth S. Kosik, Harriman Professor of Neuroscience, reveals some very unique evolutionary innovations in the primate brain.
In a study published online today in the journal Neuron, Kosik and colleagues describe the role of microRNAs—so named because they contain only 22 nucleotides—in a portion of the brain called the outer subventricular zone (OSVZ). These microRNAs belong to a special category of noncoding genes, which prevent the formation of proteins.
"It's microRNAs that provide the wiring diagram, dictating which genes are turned on, when they're turned on and where they're turned on," said Kosik, who is also the co-director of UCSB's Neuroscience Research Institute and a professor in the Department of Molecular, Cellular and Developmental Biology. "There's a core set with which all kinds of really complex things can be built, and these noncoding genes know how to put it together."
The researchers were looking for these noncoding genes, Kosik continued, because as organisms become more complex through evolution, the number of these noncoding genes has greatly expanded. "But the coding genes—the ones that make proteins—have really not changed very much," he said. "The action has been in this noncoding area and what that part of the genome is doing is controlling the genes."
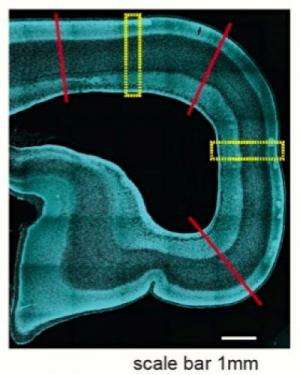
Many of the microRNAs that Kosik's team found and subsequently sequenced are newly evolved in primates. The work showed that these tiny control elements were overrepresented in the OSVZ of the developing macaque brain tissues they analyzed. The tissue samples were provided by a lab at the Stem-cell and Brain Research Institute near Lyon, France, headed by research director and co-author Colette Dehay.
Study results indicate that the appearance of the OSVZ is very much associated with the invention of new microRNAs. "There might be some relationship—although we can't prove it—between the invention of some of these new noncoding genes, microRNAs, and the appearance of a new structure, the OSVZ," Kosik said. "Trying to connect an anatomical, morphological invention with genes is very difficult, but our work shows a possible molecular basis for the tools that were needed to build this novel structure."
The analysis found that these new microRNAs target old genes, many involved in the cell cycle, which is responsible for cell division (mitosis). "Nearly all cells throughout evolution have a cell cycle," Kosik explained. "We can watch the evolutionary process at a very molecular level, see what is novel and how molecular innovation affects what already exists, like the cell cycle. When new things are invented in evolution, they have to be integrated with what already exists.
"What I find fascinating is that the whole ancient cellular mechanism of cell division still has enough evolutionary space left to make something new and to make something new that's really complex," he added. "The OSVZ gave rise to primates' expanded brains and to the cells that ultimately brought us Shakespeare."
According to Kosik, the microRNAs he studied are a melding of molecular and anatomical information. "Some of the genes we found that are the targets of these new microRNAs are also involved in certain human developmental disorders that are genetic," he said.
"One place we would like to go with this information is to explore pathways that may be manipulated to help patients in some way," he said. "We know people with developmental disorders may be missing a critical gene involved in brain formation and wiring, so maybe if we understood the control of those genes—as these new data are pointing to—we might be able to do something that could be applied to a human condition."