Glaucoma drug helps women with blinding disorder linked to obesity
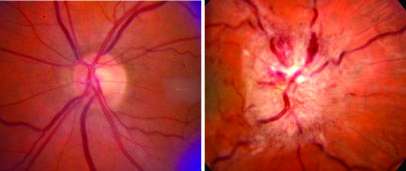
An inexpensive glaucoma drug, when added to a weight loss plan, can improve vision for women with a disorder called idiopathic intracranial hypertension (IIH), according to a study funded by the National Institutes of Health.
IIH, also called pseudotumor cerebri, predominantly affects overweight women of reproductive age. An estimated 100,000 Americans have it, and the number is rising with the obesity epidemic. The most common symptoms are headaches and visual problems, including blind spots, poor side vision, double vision and temporary episodes of blindness. About 5-10 percent of women with IIH experience disabling vision loss.
"Our results show that acetazolamide can help preserve and actually restore vision for women with IIH, when combined with a moderate but comprehensive dietary and lifestyle modification plan," said Michael Wall, M.D., a professor of neurology and ophthalmology at the University of Iowa in Iowa City.
The trial was funded by NIH's National Eye Institute, and coordinated by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC). The results were published today in the Journal of the American Medical Association, and will be presented on May 2 during the Clinical Trials plenary session of the American Academy of Neurology meeting in Philadelphia.
Acetazolamide (Diamox) is best known as a glaucoma drug. It has been commonly prescribed for IIH, but without much evidence that it helps. The IIH Treatment Trial tested the benefits of acetazolamide plus a weight loss plan versus the weight loss plan with a placebo pill, over six months. Patients in both treatment groups had improved vision, but those receiving the drug had the greatest improvement. All patients were allowed to take headache medications throughout the trial, and both groups experienced a similar reduction in headache.
"The vision problems associated with this condition can be extremely debilitating, at significant cost to patients and the health care system. Yet there are no established treatment guidelines. We made it a priority to develop an evidence-based treatment for helping patients keep their vision," said Eleanor Schron, Ph.D., director of clinical applications at NEI.
IIH is named for one of its key physical findings—an increased pressure within the fluid-filled spaces inside and around the brain. This in turn can cause swelling and damage to the optic nerves that connect the eyes to the brain. A 5-10 percent weight reduction appears to improve symptoms for many patients, but can be difficult to achieve and maintain. Acetazolamide is known to reduce fluid production in the brain, and is often used as an add-on therapy. In severe cases, surgical procedures may be used to relieve pressure on the optic nerve.
The dosing and results with acetazolamide vary. In high doses, the drug can produce side effects including fatigue, nausea, tingling hands and feet, and a metallic taste, usually triggered by carbonated drinks. British researchers completed a trial of the drug for IIH in 2011, but the results were inconclusive.
The NIH-funded trial involved 161 women and four men with IIH and mild vision loss, who were enrolled at 38 sites. At enrollment, their average body mass index (BMI) was about 40. A BMI of 30 or greater is considered obese. All participants were put on a weight loss plan to trim salt and about 500 to 1,000 calories from their food intake each day, with the goal to lose 6 percent of their starting weight. They were provided with a weight loss coach and some simple low-cost exercise equipment. This included a step counter and a resistance band, a piece of rubber tubing used for strength training. About half the participants were randomly assigned to receive acetazolamide. The drug was given at 1 gram daily for the first week and increased by a quarter gram each week, to reach the maximally tolerated dosage, or up to 4 grams daily. The other half of participants received a placebo in gradually increasing dosages.
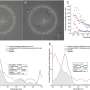
After six months, both groups had improved scores on visual field tests, a measure of side or peripheral vision. Participants on acetazolamide improved by about twice as much as those on placebo. Compared to weight loss alone, the drug also helped reduce swelling of the optic nerve. The drug-weight loss combination also led to greater improvements in daily function and quality of life compared to weight loss alone, based on the NEI Visual Functioning Questionnaire.
In the placebo group, there were six treatment failures—defined as a substantial worsening of vision that required withdrawal from the trial. There was one treatment failure in the acetazolamide group.
Seven people on acetazolamide and one person on placebo stopped taking their assigned study medication because of perceived side effects. Three people on placebo were admitted to the hospital compared to six on the drug, two of whom developed kidney stones. All side effects were reversed by stopping the drug or reducing the dosage.
"This study provides a much-needed evidence base for using acetazolamide as an adjunct to weight loss for treating IIH," said Dr. Wall. "The drug has been around since the 1950s, and prior studies have found varying degrees of efficacy. One strength of our study is that we slowly introduced patients to the highest tolerated dose, in an attempt to maximize efficacy while limiting its side effects."
Another strength of the study was the weight loss program, he said. The New York Obesity Nutrition Research Center designed the program to achieve moderate, sustainable weight control with an emphasis on changing lifestyle, as opposed to just dieting.
The trial will follow participants for five years to gauge whether they're able to maintain a healthy weight and control their symptoms over the long term.
Dr. Wall serves as the trial director. NORDIC is chaired by Mark Kupersmith, M.D., who is director of neuro-ophthalmology services at the New York Eye and Ear Infirmary. The NORDIC data coordination and biostatistics center is directed by Karl Kieburtz, M.D., M.P.H., a professor of neurology and community and preventive medicine at the University of Rochester in New York, and by Michael McDermott, Ph.D., a professor of biostatistics and neurology, also at Rochester.
More information: Wall M et al. for the NORDIC IIH Study Group. Effect of Acetazolamide on Visual Function in Patients with Idiopathic Intracranial Hypertension and Mild Visual Loss: The Idiopathic Intracranial Hypertension Treatment Trial. Journal of the American Medical Association, April 2014. DOI: 10.1001/jama.2014.3312