Trial identifies subset of breast cancer patients most likely to benefit from Neratinib
The I-SPY 2 trial identified a neo-adjuvant regimen containing the investigational drug neratinib and standard chemotherapy to be beneficial for hormone receptor (HR)-negative, HER2-positive primary (non-metastatic) breast cancer patients, according to data presented here at the AACR Annual Meeting 2014, April 5-9.
"The I-SPY 2 trial is a randomized phase II clinical trial for women with newly diagnosed stage 2 breast cancer," said John W. Park, M.D., professor of medicine at the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco. "The trial tests whether adding investigational drugs to standard chemotherapy in the neoadjuvant setting is better than standard chemotherapy alone, and the goal is to match investigational regimens with patient subsets on the basis of molecular characteristics [referred to as biomarker signatures] that benefit from the regimen.
"We were pleased that the algorithm used for the predictions functioned so well and that it actually stopped assigning neratinib to patient subgroups which were not benefiting from the drug while at the same time increasing its assignment to patient subgroups which were benefiting from the drug," added Park.
A Bayesian algorithm used in this trial identified that the neratinib-containing regimen had accrued sufficiently to "graduate" based on its prediction that this regimen would be highly likely to succeed in a phase III trial in this same subset of patients.
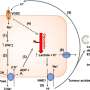
The trial is conducted using adaptive randomization: A patient with a particular subtype of breast cancer who enters the trial is preferentially assigned to treatment regimens that are performing better in patients with the same subtype of breast cancer.
Patients who have stage 2 breast cancer with a tumor size of at least 2.5 cm, and are considered to be at high risk for early breast cancer recurrence when evaluated by a test called MammaPrint, are eligible for this trial. The primary endpoint of this study is pathological complete response (pCR) in breast and lymph nodes at the time of surgery.
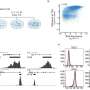
The algorithm randomly assigned 115 patients to the arm of the trial that contained neratinib, a pan-HER inhibitor. The rates of pCR on the neratinib arm were compared with those of 78 patients who were concurrently randomized to the control arm containing standard chemotherapy. These comparisons were made for 10 biomarker signatures prospectively defined by categories of HR, HER2, and MammaPrint.
The investigators concluded that the probability that the neratinib-containing regimen has a higher rate of pCR than control therapy in HR-negative, HER2-positive breast cancer (one of the 10 biomarker signatures) is 95 percent and its predictive probability of success in a future, randomized, 300-patient phase III trial is 79 percent. The algorithm also predicted this drug combination is likely to be beneficial for all HER2-positive breast cancer patients (a second of the 10 biomarker signatures), with the probability of superiority over standard therapy and the probability of success in a phase III trial being 95 percent and 73 percent, respectively.
The I-SPY 2 trial's adaptive statistical design was developed by the overall principal investigators for the I-SPY trial, Laura J. Esserman, M.D., MBA, professor of surgery and radiology and director of the Carol Frank Buck Breast Care Center at UCSF Helen Diller Family Comprehensive Cancer Center, and Donald A. Berry, Ph.D., professor of biostatistics at The University of Texas MD Anderson Cancer Center and founder of Berry Consultants.
"Traditional phase III clinical trials are large because they include patients who do not benefit from the experimental therapy, and they persist in randomizing such patients in the trial to the very end," said Berry. "Clinical trial designs have to change to keep pace with the amazing advances being made in biology. I-SPY 2 may not be the final answer, but so far it has been successful in expanding the boundaries of clinical trials, making them more patient-friendly while preserving their scientific integrity," said Esserman.
I-SPY 2 is sponsored by QuantumLeap Healthcare Collaborative, a 501(3)C charitable foundation, dedicated to accelerating health care solutions. QuantumLeap shares a unique partnership with the Foundation for the National Institutes of Health Biomarkers Consortium, which sponsored the trial until 2013, and continues to manage the intellectual property that emerges from it.