CDC urges anti-HIV pill for people at high risk of infection
(HealthDay)—People deemed to be at high risk for contracting HIV, the virus that causes AIDS, should take anti-HIV medicines that seem to cut transmission risk, the U.S. Centers for Disease Control and Prevention announced Wednesday.
If used consistently, this approach, called pre-exposure prophylaxis (PrEP), has been shown to reduce HIV infection rates in prior studies by as much as 90 percent, the CDC noted.
"HIV infection is preventable, yet every year we see some 50,000 new HIV infections in the United States," CDC director Dr. Tom Frieden said in a news release from the agency. "PrEP, used along with other prevention strategies, has the potential to help at-risk individuals protect themselves and reduce new HIV infections in the United States."
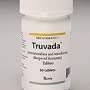
The new guidelines are tied to the 2012 approval by the U.S. Food and Drug Administration of a combo drug called Truvada for use as PrEP, along with safe sex practices.
"While a vaccine or cure may one day end the HIV epidemic, PrEP is a powerful tool that has the potential to alter the course of the U.S. HIV epidemic today," Dr. Jonathan Mermin, director of CDC's National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said in the news release.
The new guidelines say that the use of the PrEP regimen should be considered by the following groups:
- Anyone involved in an ongoing relationship with a person who is already infected with HIV;
- Any gay or bisexual man who has had sex without a condom or who has been diagnosed with a sexually transmitted infection within the past six months, and is not in a mutually monogamous relationship with someone who recently tested HIV-negative;
- A heterosexual person who does not always use condoms when having sex with people who might be at high risk for HIV (injection drug users or bisexual male partners whose HIV status is unknown) and is also not involved with an HIV-negative person in a mutually monogamous relationship;
- Anyone who has abused injected, illicit drugs over the past six months, shared needles or other equipment tied to injected drug abuse, or been in a drug abuse treatment program.
The CDC is offering PrEP providers with support to help make sure that people on the regimen adhere to it as closely as possible—always an issue, the agency said. Pills need to be taken regularly or the level of protection from PrEP drops dramatically, the CDC noted.
That's why safe sex practices— condoms, especially—remain important.
One expert in the field said that preventing HIV will always take more than a pill.
"We applaud the CDC for acknowledging the effectiveness of PrEP to reduce the risk of HIV infection among those individuals at greatest risk of HIV transmission," said Dr. Michael Mullen, professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, in New York City.
"However, it must be emphasized that PrEP cannot and should not be used alone as prophylaxis against HIV infection—rather, PrEP must be used in combination with other proven preventive measures, including practicing safe sex, being tested regularly for STDs, and knowing your and your partner's HIV status," he said.
Dr. Dawn Smith is the epidemiologist in CDC's Division of HIV/AIDS Prevention who led the development of the guidelines. She stressed that, "individuals will have to decide with their doctor if PrEP is right for them, but for some, this may offer a much-needed strategy to help protect themselves from HIV infection."
But the strategy will require teamwork, she added.
"PrEP is a new approach to HIV prevention that requires continuing collaboration between patients and providers, as effectiveness requires adherence to daily medication and regular medical visits for monitoring, counseling and testing," Smith said.
The new guidelines were published May 14 in the CDC journal Morbidity and Mortality Weekly Report.
More information: There's more on preventing HIV infection at the U.S> National Institutes of Health.
Copyright © 2014 HealthDay. All rights reserved.