Cetuximab or bevacizumab with combi chemo equivalent in KRAS wild-type MCRC
For patients with KRAS wild-type untreated colorectal cancer, adding cetuximab or bevacizumab to combination chemotherapy offers equivalent survival, researchers said at the ESMO 16th World Congress on Gastrointestinal Cancer in Barcelona.
"The CALGB/SWOG 80405 trial was designed and formulated in 2005, and the rationale was simple: we had new drugs —bevacizumab and cetuximab— and the study was designed to determine if one was better than the other in first-line for patients with colon cancer," said lead study author Alan P. Venook, distinguished Professor of Medical Oncology and Translational Research at the University of California, San Francisco, USA.
The CALGB/SWOG 80405 trial studied patients whose tumours were KRAS wild-type at codons 12 and 13. Patients received mFOLFOX6 or FOLFIRI at the discretion of their doctor and were randomised to cetuximab (578 patients) or bevacizumab (559 patients).
"There was no meaningful difference in outcome between treatment arms," said Venook. "In both arms patients lived close to 30 months. About 10% of patients lived more than 5 years. Overall patients did much better than anticipated and it was indifferent to the type of treatment."
Because almost 75% of the patients received mFOLFOX6 as the chemotherapy, the interaction between the experimental drugs and chemotherapy will be limited, but an analysis is underway. The investigators are also conducting molecular analyses that may identify subsets of patients who did better or worse on either treatment.
Commenting on the data, ESMO spokesperson Dirk Arnold, Director of the Department of Medical Oncology, Tumour Biology Centre in Freiburg, Germany, said: "This was a long awaited phase III trial with a head-to-head comparison of two different molecular approaches: epidermal growth factor receptor (EGFR) blocking by cetuximab on one side and antiangiogenic (anti-vascular endothelial growth factor [VEGF]) inhibiting treatment with bevacizumab on the other side, both in combination with any standard first-line chemotherapy in metastatic colorectal cancer. The trial is important because the primary endpoint was overall survival. The FIRE-3 trial presented last year indicated that there may be an overall survival benefit with cetuximab but overall survival was only a secondary endpoint and data was inconclusive."
"Each of the monoclonal antibodies, in combination with standard chemotherapy, gave an overall survival of about 30 months: this is the longest overall survival in such a large trial and clearly sets the standard," Arnold continued. "We now know that using any monoclonal antibody with any standard chemotherapy in first-line treatment may give the patient the likelihood of surviving about 30 months. However there is no clear winner in terms of overall survival."
"Next we have to see the analyses of the pan RAS cohort. Then we need to find out if different subgroups benefit more from anti-EGFR or anti-VEGF treatment. The type of chemotherapy or localisation of the tumour may also play a role."
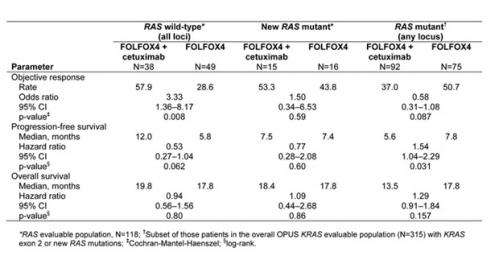
In connection with this trial, results of the now updated phase III CRYSTAL trial and phase II OPUS trial show that adding cetuximab to FOLFIRI or FOLFOX4 in the first-line treatment of metastatic colorectal cancer provides a greater benefit for patients with RAS wild-type tumours compared with the initial analysis with KRAS wild-type selected patients. Patients with RAS tumour mutations did not benefit.
Commenting on the data, Dirk Arnold said: "The CRYSTAL and OPUS trials confirm the results of the PRIME trial of FOLFOX and panitumumab, another anti-EGFR. These trials excluded all mutations in the KRAS and NRAS genes and looked at the benefit of anti-EGFRs in wild-type patients. All three trials consistently show that anti-EGFRs plus chemotherapy do better than chemotherapy alone. And anti-EGFRs in pan wild-type patients do better than anti-EGFRs in only exon 2 wild-type patients."
He added: "The only issue raising some questions is the fact that patients who bear any mutations may be at risk of a detrimental effect. So genetic testing is not only a prerequisite to ensure the maximum benefit, it is also needed to ensure that we do not harm patients by treating them."
More information: References
[1] Abstracts from the 16th ESMO World Congress on Gastrointestinal Cancer are published in Annals of Oncology, Volume 25 suppl 2 June 2014 (Ann Oncol 2014 Jun; 25(Suppl 2): 1-117) annonc.oxfordjournals.org/content/25/suppl_2.toc
[2] Abstract presentation: Saturday, 28 June 2014, 10:50 hrs, Session XVIII: Prevention and screening of colon cancer













