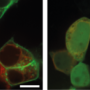
Discovery reveals how measles and Nipah viruses elude immune detection and activation
Sensors of microbial infections are the immune system's first line of defense. These molecules are the sentries guarding against dangerous invaders. Once they spy a threat, they sound an alarm, marshaling other defenders to mount a search-and-destroy mission.
Viruses have evolved ways to avoid detection by these immune sensors. The pathogens elude exposure and also mute messages that would call in the big guns of the adaptive immune response once alerted by the innate immune sensors.
Michaela Gack, Harvard Medical School assistant professor of microbiology and immunobiology, has been studying the very first steps in the immune-sensing process of viral pathogens. Last year she revealed an important role for a phosphatase known as PP1, a molecule whose chemical job is to remove phosphate groups from proteins.
She discovered that PP1 activates the early immune response by removing inhibitory phosphorylation marks on a major class of immune sensors of viral infection. Removing these inhibitory phosphates, a process called dephosphorylation, activates these immune sensors and sends the troops into battle by triggering the production of antiviral proteins called cytokines.
In uninfected cells, adding of phosphates, a process fittingly called phosphorylation, keeps these sensors in check and prevents production of infection fighters such as interferon. Normally these two processes of phosphorylation and dephosphorylation are kept in balance, fighting infection when needed but also dampening the immune response to avoid inappropriate activity, which could lead to autoimmune disease.
Gack has focused her research on the not well-characterized sensor MDA5, which has recently emerged as a key sensor for detecting a family of viruses that includes measles and Nipah virus. Measles, which was almost stamped out in developed countries until small groups of parents began objecting to vaccinating their children, poses a growing threat to vulnerable people: the very young, the very old, and people whose immunity is weak. Cases in the U.S. usually number about 50 per year, but in the first five months of 2014 the count is already up to 400, a sign that the herd immunity is waning.
Nipah virus is more deadly, but it is also geographically restricted. It is found mostly in regions of Southeast Asia where it is spread from fruit bats to people. There is no vaccine or treatment for Nipah infection.
Now Gack and collaborators from Boston University School of Medicine (BUSM) and the University of Amsterdam have discovered that measles and Nipah viruses manipulate the phosphorylation state of the immune sensor MDA5, keeping it inactive while the virus enters cells and replicates. Their findings are published in Cell Host & Microbe.
The scientists found when they infected cells with dengue or encephalomyocarditis virus, for example, PP1 removed phosphates from MDA5, allowing it to activate and setting in motion the innate immune response. But when cells were infected with measles virus, this did not occur.
"So the virus replicated undetected, and it also actively suppressed the immune system from responding," Gack said.
To learn how measles virus was interfering with the phosphorylation-dephosphorylation balance, the scientists took a closer look at a viral protein called, simply, V. The V protein of measles, as well as the related Nipah virus, is already known for enabling these two viruses to potently suppress immunity, but Gack and her team discovered precisely how it works.
They found that the V proteins of measles and Nipah viruses target the phosphatase PP1. At the tail end of the measles V protein is a defined PP1-binding motif that allows V to bind to PP1 and sequester it away from the immune sensor MDA5. If PP1 is hidden away and bound to the V protein, the sensor MDA5 is unable to do its job of sounding the alarm by inducing cytokines.
When the V protein was mutated so that it lacked its tail region containing the PP1-binding motif, the mutant virus replicated at levels a thousand-fold lower in human lung cells, showing how powerfully the immune system can respond when it senses the virus and send a storm of infection-fighting cytokines to defeat it.
Gack said her work was made possible by a collaboration with Paul Duprex at BUSM, who constructed the V mutant -carrying measles virus based on a wild-type strain of measles virus, of the clinical isolate Khartoum-Sudan. Working with a wild-type virus, rather than the typically studied version used in the weakened measles vaccine, gave the scientists a more faithful virus to study and more solid results to interpret.
The team conducted further experiments with primary human dendritic cells in collaboration with Teunis Geijtenbeck's group at the University of Amsterdam, Netherlands. Dendritic cells are immune cells that also become infected by many viruses. During measles infection, lung cells are the first to become infected, but then the virus moves on to dendritic cells and other immune cells. They concluded that the same manipulation of the phosphorylation-dephosphorylation state of the sensor MDA5 took place in these cells, too, evading immune response in the same way.
The viral strategy makes sense, Gack said.
"These are very small RNA viruses," she said. "They have very few proteins so it's logical for them to target the key player—the immune sensor—in our system."