First drug candidate from NIH program acquired by biopharmaceutical company
A drug candidate developed by researchers at the NIH's National Center for Advancing Translational Sciences (NCATS) and its collaborators to treat sickle cell disease has been acquired by Baxter International's BioScience business. The drug candidate, Aes-103, is the first specifically developed to target the underlying molecular mechanism of sickle cell disease. Baxter now will advance the clinical development activities required for regulatory approval and commercialization.
Sickle cell disease is a genetic blood disorder that affects millions worldwide, including approximately 100,000 people in the United States—among them, 1 in 500 African-Americans.
This is the first time a company has acquired a drug candidate developed with NCATS' Therapeutics for Rare and Neglected Diseases (TRND) program resources. Baxter International recently acquired AesRx, LLC, Newton, Massachusetts—the TRND program collaborator—including Aes-103. TRND and AesRx researchers worked together to develop Aes-103 through a Phase II clinical trial to evaluate safety and effectiveness. The trial data indicated that Aes-103 significantly reduced patients' pain.
"This is a wonderful example of why NCATS was created," said NIH Director Francis S. Collins, M.D., Ph.D. "The progress made thus far in the development of Aes-103 demonstrates NCATS' catalytic role in bringing together the necessary players, whether academic, nonprofit or industry, to overcome obstacles to translation and advance badly needed treatments to patients."
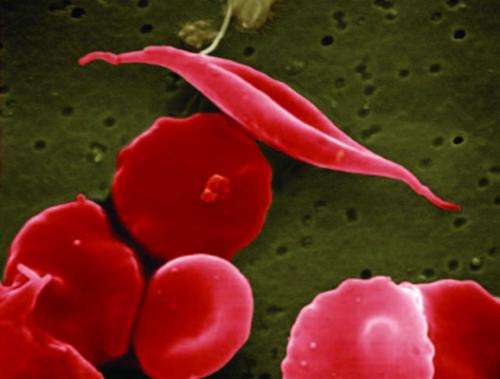
Individuals living with sickle cell disease have defective hemoglobin, the protein in red blood cells that carries oxygen. This defect causes their cells to become rigid and crescent-shaped, blocking small blood vessels and causing inflammation, pain and strokes, and decreased blood flow.
Aes-103 works by binding directly to hemoglobin and changing its structure, thereby reducing the sickling of red blood cells. This structural change may lessen sickling-related complications in patients.
Sickle cell disease disproportionately affects African-Americans and is considered both rare and neglected in the United States. African-Americans with sickle cell often face significant health disparities in clinical care. Life expectancy for people with sickle cell disease is only to mid- to late 40s.
Prior to AesRx's collaboration with TRND researchers, and despite promising data on Aes-103, the company had difficulty securing private financing because potential investors lacked interest in funding an early-stage project that was considered too risky. AesRx did not have the resources to complete preclinical and early clinical development.
Currently, the only drug approved by the U.S. Food and Drug Administration (FDA) to treat sickle cell disease is hydroxyurea, a drug initially developed to treat cancer. However, the clinical utility of hydroxyurea is limited. Many individuals with sickle cell disease either do not respond to the drug, or they may experience undesirable side effects.
"Sickle cell was the first disease to ever have its molecular cause discovered—more than 65 years ago—and now a potential treatment based on that discovery has at last been developed," said NCATS Director Christopher P. Austin, M.D. "This success validates the NCATS model, which is based on a novel collaborative approach that de-risks intervention development programs to enable private-sector investment. We look forward to applying this model to the thousands of rare diseases that are currently untreatable so that we realize the NCATS mission of getting more treatments to more patients more quickly."
TRND researchers signed a collaborative agreement with AesRx in 2010 and established a project team made up of NCATS and AesRx scientists as well as a leading sickle cell disease clinical researcher at the National Heart, Lung, and Blood Institute (NHLBI). Other key project collaborators received support through NHLBI grants, the NIH Clinical Center and its pharmacy, and NCATS' Bridging Interventional Development Gaps program. Aes-103 was licensed by AesRx from Virginia Commonwealth University, Richmond, where the compound was discovered.
"This is an important milestone for the development of this potential sickle cell disease therapeutic, and we are pleased that NHLBI researchers were able to play a role in advancing this project from the beginning," said NHLBI Director Gary H. Gibbons, M.D. "NHLBI is dedicated to advancing sickle cell disease research as a strategic priority in an effort to improve the quality of care received by patients."
In less than one year, the team completed the preclinical toxicology, chemistry, manufacturing, controls and regulatory studies necessary to support an investigational new drug (IND) application, which AesRx filed with the FDA. After IND clearance, Aes-103 moved into Phase I clinical trials in healthy volunteers and sickle cell disease patients in 2011 and into a Phase II trial in patients in 2013. The project results also helped AesRx obtain a Massachusetts Life Science Accelerator loan to support development of Aes-103.
"This project may never have reached clinical trials if not for the TRND program and its preclinical drug development expertise and novel approaches," said Stephen Seiler, AesRx's founder and former CEO. "We believe Aes-103 has the potential to be a breakthrough in the treatment of sickle cell disease. TRND's support for AesRx has enabled us to bring that potential closer to realization."
"Acquiring AesRx and this clinical development program is an important opportunity, as it complements Baxter's established relationships and expertise in treating rare and challenging blood disorders," said Ludwig Hantson, Ph.D., president of Baxter BioScience. "This investment reflects our continued focus on addressing high unmet clinical needs for patients with inadequate treatment options and no recent major clinical developments."