ReWalk receives FDA approval, clearing the way for US sales
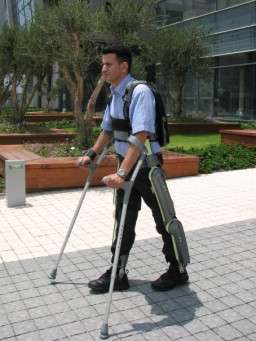
There is good news for those suffering from spinal cord injuries. The U.S. Food and Drug Administration gave its stamp of approval to ReWalk—the exoskeleton that enables paraplegics to walk. While the device has sparked other companies to make similar products, ReWalk, invented by Technion graduate Dr. Amit Goffer, is the first motorized exoskeleton to receive FDA approval.
The ReWalk Personal System is already available in Europe and Israel, but was limited in the U.S. only for use in rehabilitation and veterans' clinics. The FDA approval, announced June 26, 2014 by Argo Medical Technologies, will allow people to purchase the device for private use. Users must have upper body strength to manipulate crutches, (making ReWalk unsuitable for quadriplegics), and undergo some 15 one-hour training sessions to learn to walk. The price of the device has not officially been publicized, but news reports speculate it will cost between $65,000 - $68,000.
"This revolutionary product will have an immediate, life-changing impact on individuals with spinal cord injuries," said Larry Jasinski, CEO of ReWalk Robotics. "For the first time, individuals with paraplegia will be able to take home this exoskeleton technology, use it every day and maximize on the physiological and psychological benefits we have observed in clinical trials. This is truly the beginning of 'ReWalking' as a daily reality in the U.S."
ReWalk is an exoskeleton that straps onto the user's legs and allows paraplegics to stand, walk and even participate in marathons. It is worn with a backpack to carry its computer and battery, and is equipped with electric motors and sensors that anticipate shifts in the user's balance and send signals to the computer that initiate movement. In addition to allowing independent walking, studies have shown that users improve their cardiovascular health, slow the loss of fat tissue, build muscle mass and improve gastrointestinal functioning.
Derek Herrera, a U.S. Marine Corps Captain who was paralyzed in Afghanistan and has trained with ReWalk, will be one of the first Americans to own the device. "I see this as a milestone for people in my same situation, who will now have access to this technology—to experience walking again and all of the health benefits that come from ReWalking," he says.
ReWalk was invented after a tractor accident in 1997 left Dr. Goffer a quadriplegic. He created Argo to develop the product, which has won "best invention" awards from Popular Science and Time magazines. Currently, Dr. Goffer is at work on UPnRIDE, a product that would improve the quality of life for all wheelchair users. The new innovation is essentially an upright, wheeled device that allows wheelchair users to be fully mobile in a standing position.


















