First clinical data of therapeutic Parkinson's disease vaccine encourages continued development

AFFiRiS AG announced today at a press conference in New York results of AFF008, a Phase I clinical trial of PD01A, a vaccine against Parkinson's disease. PD01A is the first therapy against the protein alpha-synuclein, a promising Parkinson's drug target, to enter clinical testing.
The Michael J. Fox Foundation for Parkinson's Research (MJFF) supported the study with a $ 1.5 million grant, and presented at the press conference on the impact a disease-modifying therapy would have for patients. The Foundation will support a follow-up study testing a boost vaccination, the next step toward a Phase II trial.
"A treatment that could slow or stop Parkinson's progression would be a game changer for the five million worldwide living with this disease and the many more who will become at risk as our population ages", said MJFF CEO Todd Sherer, PhD. "The AFF008 trial is one of the most promising efforts toward that goal, and we're proud to support this work of AFFiRiS AG."
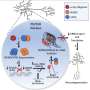
In this study, two different doses of PD01A were safe and well tolerated, meeting the primary endpoint of the trial. Secondary endpoints of the study included the induction of an alpha-synuclein-specific antibody response. A hallmark pathology of Parkinson's disease is aggregates of protein—chiefly alpha-synuclein—called Lewy bodies that accummulate in brain cells, leading to cell degeneration and cell death. Researchers hypothesize that reducing alpha-synuclein accumulation will be neuroprotective; AFFiRiS is using active immunotherapy to test that theory and develop a disease-modifying treatment.
PD01A was applied at two different doses (15 µg and 75 µg) to 12 patients per group. All received four vaccinations in monthly intervals, and all completed the study. Eight patients on best medical care, including standard symptomatic medication, served as a control group. Each patient was regularly seen and evaluated during a 12-month period.
Fifty percent of the vaccinated patients generated alpha-synuclein-specific antibodies as measured in serum samples. Additionally, vaccine-induced antibodies were detectable in cerebrospinal fluid. This induction of antibodies against alpha-synuclein is strong preliminary evidence in support of the principle of AFFiRiS' proprietary therapeutic vaccine.
Furthermore, analysis of clinical endpoints revealed a trend, consistent over all parameters, towards functional stabilization of the vaccinated groups as compared to non-vaccinated control patients. The pharmacodynamic profile of PD01A and its clinical effects will be the basis of later phase studies, should development continue.

"The safety and tolerability observed in this study, especially in a protein such as alpha-synuclein where we do not yet know its normal function, are encouraging," said Walter Schmidt, PhD, Co-founder and CEO of AFFiRiS AG. "We are grateful for the continued support of The Michael J. Fox Foundation as we progress in clinical development."
The next study will take place in Vienna, Austria and focus on assessing the immunological and clinical effects of a boost vaccination. Recruitment is expected to begin September.