August 12, 2014 feature
Fit to a T-cell: Conformal hydrogel coating could lead to immunosuppression-free islets of Langerhans transplantation
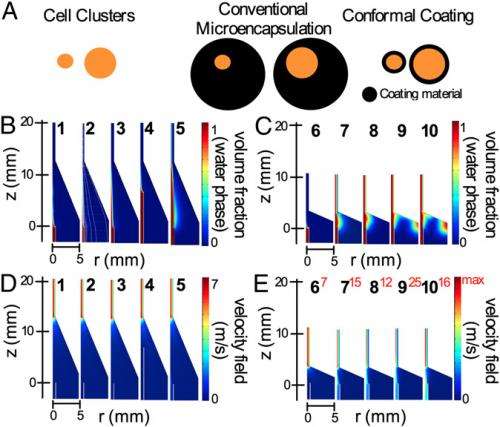
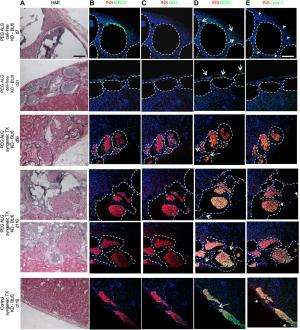
(Medical Xpress)—Type 1 diabetes is a chronic autoimmune disease in which viral infection1,2, genetics and environmental factors conspire to impact the immune system's T-cells, which then attack and destroy the pancreatic insulin-producing islets of Langerhans (also known as beta cells). There is no vaccine or cure, only management – and while a successful islet or pancreas transplant may address Type I diabetes and its associated long-term complications, the procedure requires a lifelong regimen of immunosuppressive drugs that may be only partially efficacious, have a negative impact on the islets themselves and create an increased risk of infection and cancer. Given these caveats, research has long focused on encapsulating islets in a manner that obviates the need for post-transplantation immunosuppression. That being said, current microencapsulation techniques have been shown to interfere with various islet functions, such as cellular hypoxia (lack of oxygen), delayed GSIR (glucose-stimulated insulin release), and transplant site size limitations. Recently, however, scientists at the Diabetes Research Institute, University of Miami Miller School of Medicine have designed and optimized a conformal encapsulation technique based on biocompatible permeable hydrogels, resulting in thin, complete, uniform coatings on islets of various sizes in which the coating conforms to each islet's individual shape. Moreover, syngeneic (genetically identical or sufficiently identical, and thereby immunologically compatible) diabetic mice receiving transplanted conformally coated islets achieved and maintained euglycemia – that is, normal blood glucose levels – for over 100 days without foreign body response to the encapsulating material or abnormal revascularization.
Dr. Alice A. Tomei discussed the paper that she and her co-authors published in Proceedings of the National Academy of Sciences. "Our motivation and long-term goal is making islet encapsulation successful to allow islet transplantation with no or minimal immunosuppression, which is not completely effective and is the leading cause of adverse events associated with the procedure," Tomei tells Medical Xpress. "Based on a critical review of the literature and on Dr. Hubbell's previous promising conformal encapsulation efforts6, we hypothesized that failure of traditional islet encapsulation efforts is partially due to the large size of traditional capsules, which causes impaired oxygen/nutrient diffusion and delays in insulin secretion in response to glucose challenge. Further, large capsules limit the transplantation site to the peritoneal cavity, which may not be ideal for islet transplantation due to the poor oxygen tension."
Tomei adds that their goal for the current study was to design a method for minimizing islet capsule thickness to a few tens of microns. "Since islet size ranges from 50 to 350µm, we decided to design a method that was based on shrink-wrapping the cells within the hydrogel material rather than incorporating islets within droplets of the hydrogel material with a constant diameter – the technology currently used for islet encapsulation." Furthermore, she continues, the scientists decided to avoid any direct binding of hydrogel components to the islet surface because it can prevent islet remodeling and damage the islet basal membrane, the latter being critical for protecting the islets against autoimmunity5.
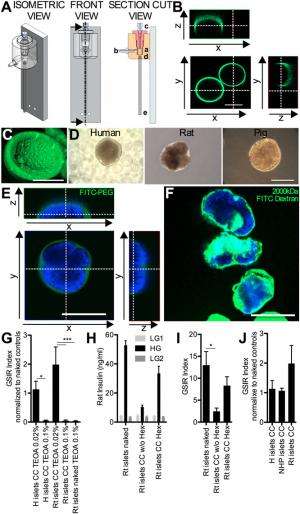
"We based our islet shrink-wrapping microfluidic system design on previous work for generation of coaxial jets3,4,8,9, as well as on conformal coating of single cells5," she explains. "Four key design challenges we encountered were defining the right combination of fluid dynamic parameters that allows conformal coating of particulates rather than generation of droplets with constant diameters; devising the process to allow PEG gel precursor polymerization after capsule formation, rather than before encapsulation within the microfluidic device; tailoring the process to islets, which are very sensitive to manipulations; and adjusting PEG hydrogel composition to maximize enclosed islet viability and function."
Tomei notes that future translational studies are needed to treat Type 1 diabetes in humans – and such studies are already in the planning stages. "In our PNAS study, we've shown that conformal coating does not compromise islet viability and function after encapsulation in syngeneic islet transplant settings in mice – and therapeutically, we've proven that conformal coating shows real potential for autologous transplants." The next steps, she continues, are to evaluate whether conformal coating provides efficient immunoisolation against allorejection and autoimmunity – studies the researchers are currently performing in small animal models with the support of the Diabetes Research Institute Foundation, the Juvenile Diabetes Research Foundation and the Leona M. and Harry B. Helmsley charitable trust (Grant 17-2012-361), and BioRep Technologies. "Additionally, we need to prove that conformal coating encapsulation is safe and efficacious in large animal models – so in parallel with efficacy and safety studies, we're scaling up procedures and devices." Tomei adds that they are also exploring novel and more effective ways to achieve local (rather than systemic) modulation of immune responses needed to prevent rejection of transplanted islets. The scientists are also investigating ways of counteracting autoimmunity by re-establishing tolerance towards beta cell autoantigens – normal tissue constituents which the immune system targets by producing autoantibodies that trigger autoimmune destruction and Type 1 diabetes.
Regarding how the intrinsic lack of size-limiting factors enabled the scientists to transplant conformally-coated islets at curative doses in sites not accessible with standard microencapsulation methods, Tomei tells Medical Xpress that standard microencapsulation methods are based on incorporating islets within droplets of the hydrogel material with a constant diameter – and in order to be able to enclose even large islets (up to 500µm in diameter), droplets need to be in the range of 600-1000µm. What happens, she explains, is that small islets – as small as 50µm – will be enclosed in a large volume of inert polymeric material. During post-implantation revascularization, blood vessels that carry oxygen and nutrients and allow glucose-sensing the insulin-secreting processes in islets will grow on the capsule surface – but the thick polymeric wall between the blood vessels and the islets form a barrier for oxygen, nutrient and glucose transport into the islets and of insulin and waste products from the islets to the blood vessels.
"Such a large transport barrier causes the center of the islets to suffer from hypoxia and central necrosis, or cell death, as well as a delayed insulin secretion in response to glucose" Tomei explains. "Additionally, since 500,000 to 1,000,000 islets need to be transplanted in humans to reverse hyperglycemia and cure diabetes, if islets are enclosed in 600µm-1000µm diameter capsules, a large volume of encapsulated material will need to transplanted, taking up hundreds of milliliters of space. Such large volumes can only be placed in the peritoneal cavity and cannot be transplanted within engineered organs like the DRI BioHub bioartificial pancreas. On the other hand," she continues, "conformal coating does not greatly increase islet volume relative to non-encapsulated, or naked, islets. Therefore, conformally coated islets can be transplanted in the same site and engineered organs where naked islets can be placed – and as mentioned, conformal coating minimizes central necrosis by not creating a barrier to the blood vessels transporting oxygen, nutrients, waste, glucose, and insulin to and from the encapsulated islet."
Given than the conformal coating thickness is determined by the geometry, viscosity ratios, interfacial tension, and flow rate ratios of the process, Tomei points out that other areas of research might benefit from their study. "The conformal coating process can be adapted to different types of cells and cell clusters, including other differentiated primary cells like neurons or renal epithelial cells, tumor and embryonic/stem cells, and different types of degradable and permanent hydrogel coating materials that will allow providing capsule performances tailored to the specific application."
More information: Device design and materials optimization of conformal coating for islets of Langerhans, Proceedings of the National Academy of Sciences, Published online before print June 30, 2014, doi:10.1073/pnas.1402216111
Related:
1Coxsackievirus B1 Is Associated With Induction of β-Cell Autoimmunity That Portends Type 1 Diabetes, Diabetes February 2014 vol. 63 no. 2 446-455
2Virus Antibody Survey in Different European Populations Indicates Risk Association Between Coxsackievirus B1 and Type 1 Diabetes, Diabetes February 2014 vol. 63 no. 2 655-662
3Formation of dispersions using "flow focusing" in microchannels, Applied Physics Letters (2003) 82(3):364-366
4Micro- and nanoparticles via capillary flows, Annual Review of Fluid Mechanics, Palo Alto: Annual Reviews, 2007:89-106.
5Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells, Proceedings of the National Academy of Sciences USA (2008) 105(9):3191-3196
6In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes, Cell Transplantation 1999 8(3):293-306
7The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human, Diabetes (2013) 62(2):531-542
8Drop and Spray Formation From a Liquid Jet, Annual Review of Fluid Mechanics (1998) 30(1):85-105
9Tip streaming from a liquid drop forming from a tube in a co-flowing outer fluid, Physics of Fluids (2006) 18(8)
© 2014 Medical Xpress
















