The first non-invasive test to diagnose fatty liver disease is awarded two patents in the US
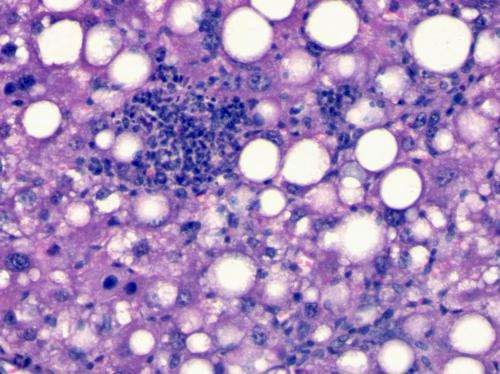
NAFLD is a progressive disease that ranges from fatty build-up (steatosis) to non-alcoholic steatohepatitis (NASH), which is inflammation around the fats. Non-alcoholic fatty liver disease is the most common liver disease in Europe and the US, and its prevalence is currently increasing in many other regions of the world, especially Asia and India.
The prevalence of steatosis and NASH in adults in the West is around 30% and 3%, respectively. NASH is the most serious manifestation of NAFLD as around 20% of NASH patients go on to develop cirrhosis within 10 years, with over a quarter of these patients subsequently developing hepatocellular carcinoma (HCC), or liver cancer, after around 10 years.
CIC bioGUNE researchers, in collaboration with OWL, CIBERehd, and eleven hospitals and research centres in Spain, France and the US developed in 2012 the first metabolomics-based serum test for non-invasive diagnosis of this disease. It is already being marketed as Owl Liver by the Basque company OWL. This test correctly classifies the disease in 94% of patients.
Owl Liver has already been awarded two patents in the US and will soon enter the markets of Brazil and Mexico, thanks to the agreements reached with the hospitals Das Clínicas (Sao Paulo) and Médica Sur (Mexico City), respectively.
According to Pablo Ortiz, CEO of Owl, "Owl Liver has become the most accurate non-invasive diagnosis test," and he believes that the fact that they have been awarded two patents in the US for these markers "increases the exclusiveness of our product in the American market and enables us to look for a licence holder who can help us to develop the product in the US".
In this sense, the Basque company has signed an agreement with the University of Virginia, which has enabled Owl to have access to samples of American patients from a world-renowned researcher, Dr Arun Sanyal, Chairman of the FDA's NASH commission. "This work has confirmed the key role of the markers that we find in our validation test," says Ortiz.
"This international experience will be added to the experience we are gaining at the Hospital Clínico in Barcelona and in the hospital network in the Basque Country that took part in the monitoring study with patients with suspected NASH, where the test can be prescribed by Social Security hepatologists. We hope that all this experience will give the test a privileged position in Spanish-American hepatology," he continues.
"But, without doubt, sales will actually increase thanks to its usage in the development of therapeutic products that are going to enter market. In this sense, we are in talks with three of the five companies that are in a more advanced stage of the clinical development of their products. One agreement with one or several of these companies would help sales to increase exponentially," he concludes.
NASH diagnosis
NASH is currently diagnosed histologically and requires a liver biopsy. A liver biopsy is an invasive and subjective procedure that entails complications (the risk of death is close to 0.01%), and is prone to sampling errors.
As well as liver biopsies, diagnostic imaging techniques such as MRI and ultrasound images measure the fat, although these procedures are not able to distinguish between simple steatosis and NASH. Because of the limitations of liver biopsies and diagnostic imaging techniques, patients with NAFLD may benefit from the metabolomics-based non-invasive method proposed to predict NASH.
The test conducted by CIC bioGUNE and the Basque company OWL is based on a study carried out between 2009 and 2011 on 467 patients who had had biopsies, with a normal histology (90) or diagnosed as having NAFLD (steatosis, 246; NASH, 131). Around 700 serum metabolites were then analysed (including amino acids, glycerolipids, phospholipids, sphingolipids, fatty acids, acylcarnitines and bile acids) using a technique called ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS).
According to CIC bioGUNE director, Prof. José María Mato, "the analysis of this large amount of metabolomic information reveals that the metabolic footprint of NAFLD depends on patients' body mass index (BMI), which means that the pathogenic mechanism of NAFLD may vary according to each patient's level of obesity".
BMI is the index that measures the weight of a person based on their height, by dividing their weight in kilos by the square of their height in metres. Over 30 is considered obese and increases the risk of developing fatty liver disease.
According to Prof. Mato, "metabolomics will enable early diagnosis of complex diseases, such as liver, cardiovascular or neurodegenerative diseases, and it will also be used to monitor the response to treatments, that is, to find out early who responds to a certain treatment and who does not, in order to identify fast those who have an adverse reaction to the medication".











