The simplistic beauty of a free radical
The study was conducted at the Center for Self-Assembly and Complexity within the Institute for Basic Science (IBS) in South Korea. Director Kimoon Kim and his team experimented with nitric oxide, a highly stable molecule of supreme importance in science. NO is highly reactive and a free radical, meaning a single, unpaired electron is present in its molecule.
Put simply NO plays the role of a mediator between elements and helps them combine. Radicals are regularly generated in many metabolic pathways. Some of these radicals can exist in a free form and subsequently interact with various tissue components resulting in dysfunction. The potential role of free radicals in the pathology of several human diseases has resulted in extensive research. However, because free radical-mediated changes are pervasive and often poorly understood, the question of whether free radicals are a major cause of tissue injury and human disease remains unanswered
Only a few organic compounds have been utilized to react with NO to form radical nitric oxide compounds and the development of such reactivity with NO using N-heterocyclic carbenes (NHCs) has not been reported to date. NHCs are not well known to react with or stabilize main group radicals and radical ions. Main group radicals, along with nitrogen, include carbon and oxygen.
The Creation of NHCNOs
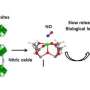
N-heterocyclic carbine nitric oxide radicals were prepared for the first time by direct addition of nitric oxide to two readily available N-heterocyclic carbenes in solution phase. The compounds, stable in air and in water, were fully characterized using X-ray crystallography and by a technique called EPR which is used to study chemical species with unpaired electrons. A functioning group of molecules from the solid molecules obtained can be thermally transferred to another NHC, suggesting potential applications to NO delivery. A light orange solid was the result of the experiment.
Although nitrous oxide was dominantly formed during chemical decomposition of the experiment, nitric oxide was also found to be thermally transferred to other N-heterocyclic carbenes. This suggests potential biological applications for NO delivery. Furthermore, this study adds another example of stable singlet carbenes acting as mimics for transition metal centers.
More information: "N-Heterocyclic Carbene Nitric Oxide Radicals", JACS, DOI: 10.1021/jacs.5b01976