Discovery prompts rethink on metals and Alzheimer's disease

Researchers at the University of Melbourne have discovered that a protein involved in the progression of Alzheimer's disease also has properties that could be helpful for human health.
The discovery helps researchers better understand the complicated brain chemistry behind the development of Alzheimer's disease, a condition that affects hundreds of thousands of Australians.
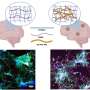
An international team of researchers, led by Dr Simon Drew at the University of Melbourne and Prof Wojciech Bal at the Polish Academy of Sciences, has revealed that a shorter form of a protein called beta amyloid, may act as a sponge that safely binds a metal that can damage brain tissue when it's in excess.
Researchers have been intensely interested in the role of beta-amyloid in the development of Alzheimer's disease. This is because clumps of the protein are formed in brains of people with the illness.
In the late 1990s, high levels of copper were discovered within these clumps. Copper is essential to health, but too much can produce harmful free radicals. Many scientists began to suspect that this copper might be contributing to the disease. They found that beta-amyloid can bind to copper indiscriminately and allow it to produce these damaging free radicals.
Closer analysis of beta amyloid protein has revealed different sizes. A good proportion of beta amyloid is missing the first three links at the start of the protein's chain-like structure.
"This short form has been overlooked by most researchers since the composition of beta amyloid was first identified 30 years ago," Dr Simon Drew explains.
"We know that the shorter form of beta amyloid is present in the diseased brain, but we now know that it is abundant in healthy brains as well.
"The small change in length makes a huge difference to its copper binding properties. We found that the short form of the protein is capable of binding copper at least 1000 times stronger than the longer forms. It also wraps around the metal in a way that prevents it from producing free radicals.
"Given these properties and its relative abundance, we can speculate this type of beta amyloid is protective. It's very different from the current view of how beta amyloid interacts with biological copper."
So far, therapies aimed at lowering the production of beta amyloid have shown only a modest ability to slow cognitive decline and the number of people affected by the Alzheimer's disease continues to grow.
Dr Drew and the team from Poland are now working to develop a method for identifying the copper-bound form of the short beta amyloid in the body.
This will enable them to screen how much copper it holds in the brain, whether it safely escorts the copper from one place to another, and how this may change in ageing and disease.
"If a beneficial role in copper balance can be established, it's still possible to have too much of a good thing," Dr Drew said.
"As the amount of beta amyloid in the brain increases during Alzheimer's disease, the shorter form can also clump together and this may interfere with its normal function. Higher levels of the short form may further enable it to soak up copper from other places where it is needed. It could be a Jekyll and Hyde scenario."
Dr Drew's research was published in Angewandte Chemie.