FDA approves iressa for EGFR metastatic lung cancer

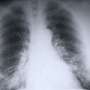
(HealthDay)—Iressa (gefitinib) has been approved by the U.S. Food and Drug Administration to treat patients with metastatic non-small-cell lung cancer (NSCLC) with a specific genetic mutation (epidermal growth factor receptor [EGFR]). A just-approved companion diagnostic test can identify patients who could benefit from this new use.
Iressa is a kinase inhibitor, a class of drugs designed to block proteins that spur development of cancer cells. The therascreen EGFR RGQ PCR Kit is a newly approved diagnostic that can help doctors detect patients with the genetic mutation who are candidates for treatment with Iressa.
Iressa was evaluated for this use in clinical trials involving 106 people with previously untreated EGFR mutation-positive metastatic NSCLC. Tumors shrank in about 50 percent of people treated with Iressa 250 mg once daily. This effect lasted an average of six months, the FDA said. Severe side effects of Iressa may include interstitial lung disease, liver damage, gastrointestinal perforation, severe diarrhea, and ocular disorders. More common side effects are diarrhea and skin reactions.
Iressa, initially approved in 2003 for a different type of NSCLC, was later voluntarily withdrawn from the market after post-approval trials failed to verify a clinical benefit, the FDA said. Iressa is marketed by Wilmington, Del.-based AstraZeneca. The therascreen diagnostic is manufactured by Qiagen Manchester Ltd., based in the United Kingdom.
More information: More Information
Health News
Copyright © 2015