FDA approves odomzo for recurring basal cell carcinoma
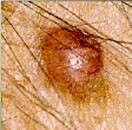
(HealthDay)—Odomzo (sonidegib) has been approved by the U.S. Food and Drug Administration to treat patients with locally advanced basal cell carcinoma that has returned despite surgery or radiation, or who are not candidates for additional surgery or radiation.
The once-daily pill is designed to inhibit the Hedgehog pathway. The drug was evaluated in a clinical study involving 66 people. Fifty-eight percent of people treated with 200 mg of Odomzo had their tumors shrink or disappear, the FDA said. The most common side effects included muscle spasms, alopecia, dysgeusia, fatigue, nausea, myalgia, diarrhea, and weight loss. More serious musculoskeletal issues also are possible.
The drug's label includes a boxed warning that it may cause death or severe birth defects in a growing fetus. Women who may become pregnant should verify pregnancy status before taking the drug. And both males and females who take Odomzo are advised to use contraception, the agency said.
"Thanks to a better understanding of the Hedgehog pathway, the FDA has now approved two drugs for the treatment of basal cell carcinoma just in the last three years," Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDA's Center for Drug Evaluation and Research, said in a statement. Erivedge (vismodegib) was approved in 2012 to treat locally advanced and metastatic basal cell carcinoma
Odomzo is marketed by Novartis, based in East Hanover, N.J.
More information: More Information
Health News
Copyright © 2015


















