Investigational HIV vaccine regimen shows encouraging results in non-human primates
Johnson & Johnson announced today that scientists at Beth Israel Deaconess Medical Center (BIDMC), Crucell Holland B.V, one of the Janssen Pharmaceutical Companies of Johnson & Johnson, and several other collaborators today published results from a preclinical study of an HIV vaccine regimen used in in non-human primates. The study, published in the online edition of Science, suggests that a "heterologous prime-boost" vaccine regimen—which first primes the immune system, then boosts the immune system to increase the response, could ultimately prove to be a strategy for protecting against global human immunodeficiency virus (HIV-1) infection.
These data form the basis of vaccine regimens that are now being evaluated in an international phase 1/2a clinical trial for safety and immunogenicity in healthy, HIV-uninfected volunteers.
"Despite great progress in HIV treatments, HIV remains one of the greatest global health threats of our time with millions continuing to be infected each year. Our ultimate goal is to develop a vaccine that prevents HIV in the first place. By Janssen collaborating with multiple stakeholders on new tools, we hope one day to help eradicate HIV," said Paul Stoffels, M.D., Chief Scientific Officer and Worldwide Chairman, Pharmaceuticals, Johnson & Johnson.
The pre-clinical study published today evaluated the protective efficacy of a "prime-boost" vaccine approach, which leverages AdVac Technology from Janssen and a trimeric envelope protein boost. Non-human primates (NHP) were first given an adenovirus serotype 26 (Ad26) vectored vaccine to prime the immune system, and then a boost of a purified HIV envelope protein intended to enhance the immune system over time. This approach is intended to increase both the magnitude of the immune response and the overall protection against subsequent viral challenge. A heterologous prime-boost vaccine regimen using a similar AdVac vector, along with an MVA-based vector, is being used in Janssen's preventative Ebola candidate vaccine regimen that is currently in Phase 1 human clinical studies.
Pre-Clinical Results Lead to Human Study
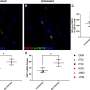
The study results show that the investigational prime-boost vaccine regimen provided complete protection from becoming infected with simian immunodeficiency virus (SIV), a virus similar to HIV that infects NHPs, in half of the vaccinated NHPs (n=12) against a series of six repeated challenges. This work also demonstrates that there is a strong link between the protective ability of the vaccine regimen and the number of antibody functions to fight the virus, so called polyfunctionality, which supports the continued development of the vaccine regimen for human use. These results have previously been presented at several international congresses.
"We are very encouraged by the results of this preclinical HIV vaccine study, and the findings lead to a clear path forward for evaluating this HIV vaccine candidate in humans," said lead author Dan H. Barouch, MD, PhD, Director of the Center for Virology and Vaccine Research at BIDMC and Professor of Medicine at Harvard Medical School.
The phase 1/2a study (HIV-V-A004) is currently enrolling 400 volunteers in the United States and Rwanda to evaluate heterologous prime-boost regimens, with sites in South Africa, Uganda and Thailand opening soon. Ongoing phase 1 clinical studies have been evaluating the safety and immunogenicity of different components which are included in the regimens to be used in this study.
The HIV-1 and Ebola AdVac-based vaccine regimens, along with Janssen's investigational candidate inactivated polio vaccine, utilize Janssen's PER.C6 production cell line technology which has the potential to reduce costs by increasing vaccine production at lower volumes, to develop, manufacture and, upon approval, commercialize vaccines.
More information: Protective efficacy of adenovirus-protein vaccines against SIV challenges in rhesus monkeys, Science, www.sciencemag.org/lookup/doi/ … 1126/science.aab3886