Improving treatment for systemic amyloidosis
A potential new approach to treat systemic amyloidosis, invented at UCL and being developed by GlaxoSmithKline (GSK), marks the start of a successful and innovative academic-industry collaboration.
The first in human clinical trial of a novel investigational drug intervention for patients with systemic amyloidosis has established proof of mechanism. Results in the first 15 patients treated with a therapeutic partnership of a small chemical molecule and a large biological molecule (an antibody) are published in the New England Journal of Medicine. Further clinical testing is in progress and a phase II trial to explore efficacy and safety is planned.
Amyloid is an abnormal protein material that accumulates in the tissues, damaging their structure and function and causing a serious and usually fatal disease called systemic amyloidosis. It is a rare disease but an important unmet medical need. Present treatments can stabilise some patients and substantially prolong life but about 20% of patients still die within 6 months of diagnosis.
The body is normally very efficient in clearing abnormal debris from the tissues but, mysteriously, this does not happen with amyloid deposits. Even when amyloid formation is controlled, the deposits are cleared very slowly if at all.
No approved treatments exist to accelerate amyloid removal. New approaches are thus urgently needed.
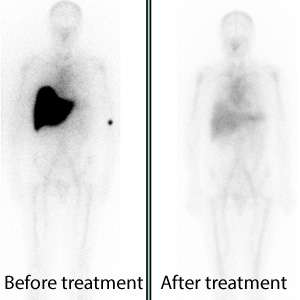
The results of the phase I study published online today showed that the antibody was generally well tolerated and produced swift clearance of amyloid from various organs. Removal of amyloid from the liver was associated with improved function. More extended follow up is required to establish functional improvement in other organs.
Professor Sir Mark Pepys FRS, Director of the UCL Wolfson Drug Discovery Unit, said: "Amyloidosis is a very challenging clinical condition. It causes serious ill health and is usually fatal, despite best efforts to support the patients and to control the underlying conditions responsible for amyloid formation. Furthermore amyloidosis can present in so many different ways that, coupled with its relative rarity, the diagnosis is often long delayed. As a result patients frequently have advanced disease before they receive any treatment. We have long recognised that measures to remove amyloid from the tissues are required and have been working on this problem for over 30 years. We were finally successful for the first time in experimental models 10 years ago. The collaboration with GSK started in 2009 and it has been a privilege to work with their expertise and resources to bring our approach into clinical testing. Seeing clearance of amyloid deposits from patients' tissues has been thrilling. It is a crucial first step on the long path towards having a medicine that could transform the outlook for people suffering from this terrible disease."
Dr Duncan Richards, Head of GSK's Academic Discovery Performance Unit, said: "Establishing proof of mechanism is a vital milestone that needs to be determined early in the path of developing a medicine so we are pleased to have this confirmed for the first time in patients. The initial results of the therapeutic intervention of CPHPC and the anti-SAP antibody are encouraging and we are now actively planning the next stages of development to better understand its potential benefits and safety in patients."
Professor Sir Mark Pepys FRS and his team in the Wolfson Drug Discovery Unit at the Royal Free Campus of UCL, have been working on amyloidosis since 1974. Their innovations in diagnosis and monitoring of amyloidosis led to establishment of the UK NHS National Amyloidosis Centre at the Royal Free Hospital in 1999. The Centre is funded directly by the Department of Health to provide diagnostic and management advice services for all patients with this disease in the UK. Some 4,000 patients visit the Centre annually, including about 1,000 newly diagnosed patients. This is probably about half the total number of amyloidosis patients in the UK.
In 2005 Pepys devised a novel strategy for amyloid removal comprising the partnership between a small chemical molecule drug and a large biological molecule
He originally developed the small molecule drug, called CPHPC, to remove a normal blood protein, called serum amyloid P component (SAP), from amyloid deposits. SAP is always present in amyloid and Pepys thought that removing it completely would help the body to get rid of the deposits. Although CPHPC treatment efficiently removes almost all the SAP from the blood it does not remove all the SAP from amyloid. Patients on CPHPC remained clinically stable but their amyloid deposits did not disappear. However, the prior administration of CPHPC uniquely enables subsequent administration of antibody to SAP that specifically targets the amyloid deposits. This approach worked well in experimental models. It successfully triggers normal mechanisms of debris clearance to efficiently remove amyloid from the tissues and restore their structure and function.
More information: "Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component." DOI: 10.1056/NEJMoa1504942













