A DNA-made trap may explain amyloidosis aggravation
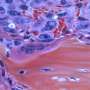
Amyloidosis is a group of clinical syndromes characterized by deposits of amyloid fibrils throughout the body. These fibrils are formed by aggregates of proteins that have not been properly folded. Deposits of amyloid fibrils are found in a number of diseases, including Alzheimer's and Parkinson's diseases and type-2 diabetes. The amyloid deposits can be localized, as in the brain of Alzheimer's patients, or found spread through the body, as in amyloidosis related to mutations in the transthyretin gene.
The clinical meaning of amyloid deposits is still poorly understood. Whereas in some patients these deposits are asymptomatic and found only by chance, in others they can damage multiple vital organs and be lethal. Previous research has suggested that what turns these apparently harmless amyloid fibrils into deadly toxic species is their breaking down into smaller pieces. How this process takes place and the identity of the key players involved are crucial questions to which a new study led by Dr Debora Foguel at the Medical Biochemistry Institute at the Federal University of Rio de Janeiro, in Brazil, provides enlightening answers.
It has been known for some time that amyloid fibrils trigger an inflammatory response, suggesting the involvement of the immune system in amyloidosis. In a paper to be published in print in November in the Journal of Biological Chemistry, the group led by Dr Debora Foguel asked whether this inflammatory response would involve neutrophils, the white blood cells that first reach a damaged site.
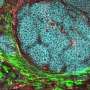
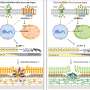
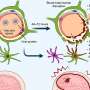
Neutrophils protect our body against microbes by releasing, at the site of infection, a DNA-made trap rich in nuclear and antimicrobial proteins, a process known as NETosis. Once caught by this neutrophil extracellular trap (NET) microbes are trapped and killed by NET components such as antimicrobial peptides e enzymes. New evidence provided by Dr Foguel's research shows that not only microbes but also amyloid fibrils can induce the release of NETs. NETs are also found at the sites of amyloid deposits in the tissues of amyloidosis patients. The study strongly indicates that amyloid fibrils are caught by NETs, which break them down into smaller fragments, mainly through the action of specific enzymes. As a side-product of this process, smaller toxic fragments that are harmful to the cells are generated.
"Our study provides the first evidence of a physiological mechanism leading to fibril fragmentation and aggravation of the disease. Thus, amyloid fibrils could be considered as a reservoir of small, toxic species," says Dr Foguel. The study entitled "Amyloid fibrils trigger the release of neutrophil extracellular traps (NETs), causing fibril fragmentation by NET-associated elastase" also shows that the extent of NET induction by amyloidosis differs among patients, which may further explain the great variability observed among amyloidosis patients.
NETs are physiologically destroyed by special enzymes capable of digesting DNA, the so-called DNAses. Indeed, some pathogens escape NETs by releasing their own DNAses when trapped. A question now remains whether amyloidosis patients are somehow incapable of disassembling these NETs when they are no longer needed, allowing them free rein and the breakdown of the amyloid fibrils into smaller toxic pieces.
The study's results have clear implications for the etiology of amyloidosis, an often-deadly disease against which little progress has been made in recent years.
More information: www.jbc.org/content/early/2012 … 369942.full.pdf+html