Newly identified mechanism solves enduring mystery of key element of cellular organization

St. Jude Children's Research Hospital scientists have discovered evidence of a mechanism at the heart of amyotrophic lateral sclerosis (ALS) and related degenerative diseases. The research appears in today's edition of the journal Cell and highlights a possible new treatment strategy for the devastating disorders.
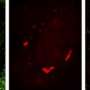
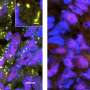
The study focused on usually short-lived compartments called stress granules that form in cells under stress. Stress granules are just one type of the membrane-less structures or organelles that assemble as needed to handle various cell functions and then rapidly disperse. Until now, however, the mechanism underlying stress granule formation was poorly understood.
Stress granules are also tied to degenerative disorders such as ALS, which is also known as Lou Gehrig's disease. Genes encoding the protein components of stress granules are often mutated in patients with ALS and other diseases. These same proteins accumulate in thread-like deposits called amyloid fibrils in the nerve and muscle cells of patients with ALS, frontotemporal dementia (FTD) and inclusion body myopathy (IBM). But the unifying mechanism was a mystery.
"This study provides the mechanism that links stress granules, toxic fibrils and disease," said co-corresponding author J. Paul Taylor, M.D., Ph.D., a Howard Hughes Medical Institute (HHMI) investigator and chair of the St. Jude Department of Cell and Molecular Biology. "In addition to advancing our understanding of fundamental cell biology, the results have spurred interest in developing drugs that target the stress granule assembly process."
The disordered segment or "tail" of hnRNPA1, a protein that is sometimes mutated in ALS and related disorders, was the key to unlocking the connection among stress granules, fibrils and disease, said co-corresponding author Tanja Mittag, Ph.D., an assistant member of the St. Jude Department of Structural Biology. hnRNPA1 is an RNA-binding protein involved in stress granule formation.
Proteins are long strings of amino acids that usually fold into specific 3-D structures. hnRNPA1 belongs to a subset of proteins with an amino acid arrangement that prevents folding of one end of the protein, which allows hnRNPA1 to adopt a variety of conformations.
In this study, researchers showed that under certain conditions related to temperature, salt and protein concentrations, hnRNPA1's disordered tail prompts the protein to condense into liquid droplets through a process called liquid phase separation. The droplets have properties similar to stress granules, including the ability to fuse and grow.
Liquid phase separation is at work in a wide range of settings, including when oil and vinegar separate in salad dressing. Until recently, however, the process was not believed to play a role in normal cell function. This study is the first to link liquid phase separation to stress granule assembly.
"It is amazing to find out that proteins like hnRNPA1 have appeared in nature to mediate liquid phase separation under normal physiological conditions," Mittag said. "The long disordered tails in these proteins enable membrane-less compartmentalization in cells. In addition, liquid phase separation is probably important for a whole range of fundamental biological processes."
Working in the laboratory, researchers also showed how the mutations likely contribute to disease. When hnRNPA1 with mutations in the disordered tail underwent liquid phase separation and concentrated into droplets, the protein almost immediately formed amyloid-like fibrils. If the mutant protein did not concentrate into droplets, however, toxic fibrils did not form.
The finding also suggests how mutations in genes like VCP promote disease. Previous research from Taylor and his colleagues have linked mutations in the gene to ALS and related disorders. VCP plays a role in dismantling stress granules when they are no longer needed. By allowing stress granules to persist, this study suggests that VCP mutations increase the likelihood that amyloid-like fibrils will form and spread.
"Rather than attempting to target each disease-causing mutation, these findings have generated interest in developing drugs that target the stress granule assembly process," Taylor said.
Currently there are no effective therapies for ALS, FTD or IBM, which are associated with a variety of neurological changes as well as muscle weakness and paralysis that affects walking, swallowing and breathing. Government agencies and advocacy groups estimate that 30,000 U.S. residents have ALS and that FTD accounts for about 10 percent of the 5.3 million Americans with dementia. IBM is one of several related disorders that, combined, affects about 50,000 residents.