Stem cell trial success could lead to new heart disease therapies

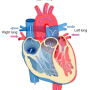
The key objective of the EU-funded CARE-MI project, which was completed in September 2015, was to evaluate the safety and efficacy of AlloCSC-01, a suspension of allogeneic human cardiac stem cells (CSCs) in patients who have suffered a heart attack. This serious condition occurs when blood stops flowing to a part of the heart, causing damage to the heart muscle.
In this way, the CARE-MI project has developed a new approach to limit tissue damage based on the activation of the heart's natural repair mechanisms in response to damage. The success of the project trials will enable the researchers to perform further analyses on stem cell treatments, with final results expected in the first half of 2017.
The hope is that AlloCSC-01 could become a revolutionary new approach to preventing cardiac disease and the onset of chronic heart failure (CHF) after a heart attack, boosting patient care and leading to a new market in stem cell therapies. In the US and Europe alone, around 1.5 million heart attacks are treated annually.
Initial recovery after a heart attack is often followed by chronic heart failure. This is partly because current therapies, while initially successful, are unable to regenerate damaged tissue. As chronic heart failure is responsible for an annual mortality rate of 18 %, the project team saw a clear need to find treatments capable of tissue regeneration.
Various cell therapies have already been proposed and tested but have proven to be marginally effective. Furthermore, the cost and the complexity of these clinical procedures make these therapies unsuitable for treating the large number of patients that need affordable and readily available products.
The CARE-MI team therefore wanted to drastically improve on these current heart disease treatments. A total of eight clinical centres of reference in cardiology participated in project trials, led by Prof. Fernández-Avilés of Hospital Gregorio Marañón in Madrid, Spain, and Prof. Stefan Janssens of KU Leuven, Belgium.
The project team built on recent research that found that heart muscles contain pluripotent cells. These cells act like endogenous cardiac stem cells (eCSCs), which are capable of anatomical and functional regeneration. The project then focused on the clinical development and testing of therapies to activate eCSCs. The ultimate objective has been to make these products biocompatible, affordable, readily available and compliant with regulatory standards.
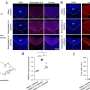
Significant inroads have been made by the CARE-MI consortium with regard to good manufacturing practice, accredited product development and testing. Allogeneic cell batches (i.e. from different human donors) were used for pre-clinical and stability tests and results confirmed their robustness and bio-equivalency.
Furthermore, extensive testing also confirmed their anti-inflammatory and hypo-immunogenic properties as well as their capacity to promote healing. Safety and bio-distribution tests were also shown to be highly promising with no major adverse effects. The team also succeeded in optimising dosing, timing and delivery methods.
A new cell production platform is now being constructed, which will enable researchers to move onto a more advanced clinical stage and ultimately bring these therapies to market.
More information: For further information please visit the CARE-MI project website: www.caremiproject.eu/