Scientists discover a trigger of Alzheimer's disease
A group of the Lomonosov Moscow State University scientists, together with their colleagues from the Institute of Molecular Biology, Russian Academy of Sciences and the King's College London, determined the mechanism of Alzheimer's disease development and possibly distinguished its key trigger. Their article was published in Scientific Reports.
"Alzheimer's disease is a widespread degenerative damage of central nervous system leading to a loss of mental ability. Until now, it was considered incurable," says Vladimir Polshakov, the leading researcher, MSU Faculty of Fundamental Medicine. Now that scientists have distinguished the mechanism responsible for Alzheimer's development, they have explored some new chemical compounds that may work as an efficient cure.
Several hypotheses are dedicated to Alzheimer's disease development. One of the most common is the so-called amyloid hypothesis.
Amyloids (to be precise, beta-amyloid peptides) are molecular constructions of a protein type; in their normal healthy state, they protect brain cells. After fulfilling their function, they are cleared by proteases, the cleaning enzymes that cut all the used protein elements into harmless 'slags' that are further reclaimed or removed from a body. However, according to the amyloid hypothesis, at some point, something goes wrong, and amyloids start aggregating and hence getting out of the reach of proteases' cutting blades. However, the early stage of a beta-amyloid transformation into harmful organic products is mostly unexplored.
"We knew, for example, that a crucial role in initiation of such processes is played by ions of several transition metals, notably zinc," says Vladimir Polshakov. "Zinc actually conducts a number of useful functions in a brain, though in this case, it is [...] an initiator of a cascade of processes, leading to Alzheimer'sdisease. However, it remained unclear what exactly happens during interaction of zinc ions with peptide molecules, or which amino acids bind zinc ions, and how such interaction stipulates a peptide aggregation. We set a goal to clarify at least some of those questions."
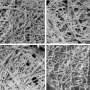
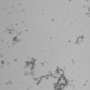
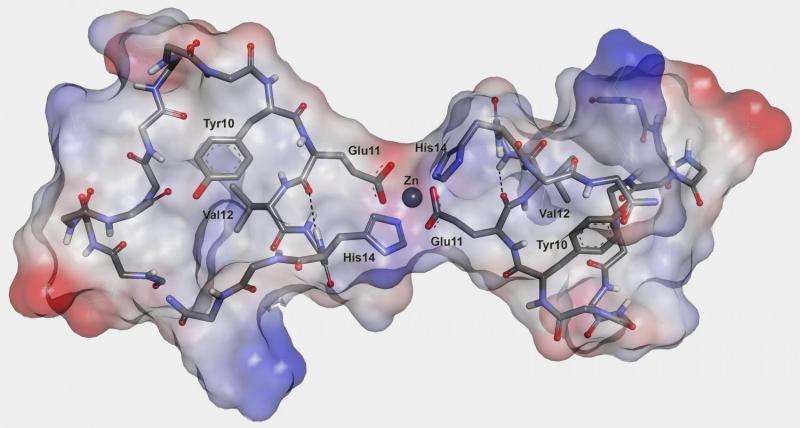
Scientists studied various pathogenic beta-amyloid peptides and their so-called metal binding domains—relatively short peptide regions capable of binding metal ions. The researchers used a number of experimental techniques, including nuclear magnetic resonance (NMR) spectroscopy, which was used to determine the structure of the forming molecular complexes. Some spectra requiring higher sensitivity were additionally measured in London. According to Polshakov, the choice of the studied pathogens was "partly luck." One of the specimens was the product of a so-called "English mutation" peptide, similar to a common beta-amyloid peptide, but with one amino acid substitution. Using NMR spectroscopy, scientists determined the chemical processes and structural changes that occur when peptide molecules interact with zinc ions and undergo further aggregation.
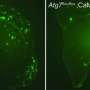
The second pathogen was an isomerized beta-amyloid peptide. One of its amino acid residues, aspartic acid, had a particular atomic position. Such isomerisms happen spontaneously, without help of any enzymes, and are related to the aging processes, another influential factor of Alzheimer's disease. Fellow biologists from the Moscow's Institute of Molecular Biology showed recently that administration of an isomerized peptide to transgenic mice led to an accelerated formation of amyloid plaques. With the presence of zinc ions, a metal binding domain of the isomerized peptide aggregated so quickly that the forming structures were hard to detect. Despite all the differences in processes occurring to the "English mutant" and isomerized peptides in the presence of zinc ions, initial stages of these transformations were similar. The trigger happened to be the same—a role of a pathogenic aggregation's seed was in both cases played by initially formed peptide dimers, i.e. two peptide molecules connected to each other with help of zinc ion. Such dimers were also detected in normal human peptides, and the difference in all the studied forms could be explained by the speed of formation of corresponding dimer and its proneness to a further aggregation.
Based on their findings, the researchers proposed the mechanism of zinc-controlled transformation of a peptide protector into a peptide killer. That mechanism, scientists note, explains multiple experimental data from previous research. Researchers also hope that their discovery could help to produce new medicine to block beta-amyloid peptide aggregation stipulated by zinc ions.
More information: Andrey N. Istrate et al. Interplay of histidine residues of the Alzheimer's disease Aβ peptide governs its Zn-induced oligomerization, Scientific Reports (2016). DOI: 10.1038/srep21734