Long-term memory has back-up plan, researchers find

A team of scientists has identified the existence of a back-up plan for memory storage, which comes into play when the molecular mechanism of primary long-term memory storage fails.
Previous work had shown that mice engineered without an enzyme crucial to long-term memory storage could still form such memories, creating a controversy that a team of scientists has now resolved with the new research, which appears in the journal eLife.
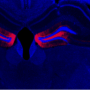
The research focused on an enzyme made in nerve cells—PKMzeta. In a series of experiments, they confirmed that while the enzyme is crucial to long-term memory in normal mice, the mice engineered without PKMzeta still form long-term memories because they deploy an alternative, previously silent memory-storage method.
"Mice missing the PKMzeta enzyme essential for long-term memory are able to recruit a back-up mechanism for long-term memory storage," explains André Fenton, a professor in NYU's Center for Neural Science and one of the paper's co-authors. "The question now is: how does PKMzeta function and what is the mechanism of its interaction with the PKCiota/lambda backup mechanisms?"
Previous research has found PKMzeta plays an important role in long-term memory storage, which scientists believe depends on the persistent strengthening of the connections between nerve cells. Specifically, the enzyme is made during the strengthening of these connections, and it remains in place so long as the links remain strong.
Notably, studies examining the role of PKMzeta's in memory found that when the enzyme's function was weakened in rodents,after they formed long-term memory, the animals could no longer remember diverse types of memories depending on diverse parts of the brain, suggesting that PKMzeta is a general memory storage mechanism.
But, recently, the importance of PKMzeta was questioned by experiments on genetically engineered "knockout" mice in which the gene that makes PKMzeta was deleted. Without PKMzeta, the knockout mice could still strengthen connections between nerve cells and still learn and remember.
While some took these results as evidence that PKMzeta's role had previously been overstated, such studies did not consider the possibility of a "back-up" mechanism for memory that takes over when PKMzeta is removed. So the question remained: Is PKMzeta unimportant for memory or, in its absence, is a back-up mechanism deployed?
To address this matter, Panayiotis Tsokas, a research professor in Todd Sacktor's lab at SUNY Downstate Medical Center, Fenton, and their colleagues tested both the "PKMzeta is unimportant" and "PKMzeta is compensated" hypotheses. To do so, they used a piece of modified DNA as a drug to block the formation of PKMzeta. If another molecule or molecules act as a back-up mechanism for PKMzeta, the scientists reasoned, the new drug would block the formation of memory in normal mice, but would have no effect on memory in the knockout mice that cannot make PKMzeta—the drug would have nothing to work on.
The results supported the PKMzeta is compensated hypothesis—the formation of memories normal mice was disrupted while that for the knockout mice was not, confirming the importance of PKMzeta, but also pointing to the presence of a back-up mechanism, which they identified involves PKCiota/lambda, the most closely related molecule to PKMzeta.
More information: Panayiotis Tsokas et al, Compensation for PKMζ in long-term potentiation and spatial long-term memory in mutant mice, eLife (2016). DOI: 10.7554/eLife.14846