Shedding new light on protein aggregates and the diseases they cause
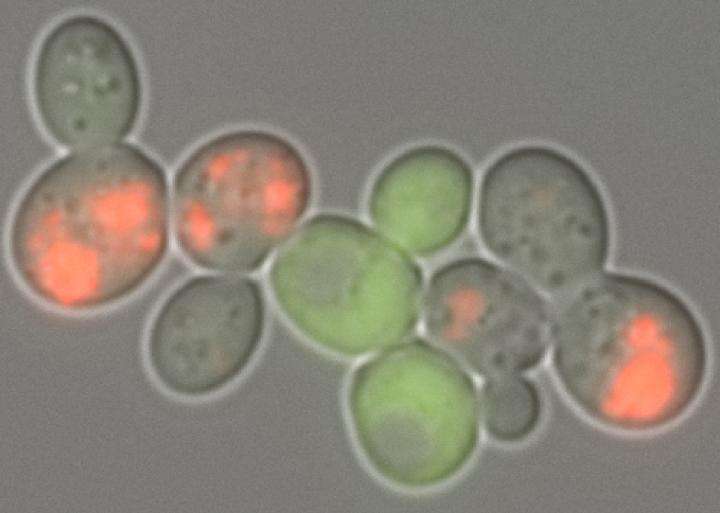
Researchers from the Massachusetts Institute of Technology and Boston University have developed a system capable of quickly screening millions of yeast cells to measure protein aggregates. Proteins regulate all of the processes that keep cells alive, but when misfolded they can clump into large aggregations, a phenomenon associated with diseases including Alzheimer's, Huntington's and Parkinson's.
The team's high-throughput method allows researchers to quickly screen for genes, drugs, mutations or new conditions that influence protein aggregates, offering new ways to explore their causes and potential new therapies.
"Part of the impetus for our work is that these protein aggregations have been very difficult to study and track quantitatively," said Greg Newby, a doctoral student at the Whitehead Institute for Biomedical Research at MIT, who performed the research. "With our system, we can rapidly measure a huge population of cells and sensitively detect even rare cells that contain protein aggregates, or have dissolved protein aggregates."
Newby will present the tool, called yeast Transcriptional Reporting of Aggregating Proteins, or yTRAP, at The Allied Genetics Conference hosted by the Genetics Society of America on July 16.
Protein aggregates form when tens to thousands of unstructured proteins clump together. Though seen in many types of cells, their causes and effects are not well understood. In addition to a number of serious neurodegenerative diseases, aggregates are associated with prion diseases, a class of disorders in which malfunctioning proteins act as infectious agents. At the same time, some protein aggregations have been associated with beneficial functions, such as facilitating the formation of memories and protecting cells from viruses.
To create the new system, Newby and his colleagues developed a synthetic, or man-made, gene that causes a cell to alter its fluorescence when a protein of interest accumulates into a clump. They inserted the gene into yeast to create a population of cells that can be rapidly and automatically screened for aggregation.
Existing methods used to detect protein aggregations have limited research applications because they are labor-intensive, cannot be used on living cells or do not reliably produce quantitative data.
Newby said the team's new system is ready to deploy for a variety of research purposes. "While there are always areas where we could consider expanding sensor capabilities, I think we're at a point now where anybody could use our system to track the aggregation of proteins of interest, or to screen for drugs that modulate those aggregations," he said.
The team applied their tool to study the protein aggregates involved in Huntington's disease, a devastating and incurable neurodegenerative disease. A region of the genome called huntingtin is expanded in many people with the disease; the length of this region is known to affect the timing of disease onset. The researchers were able to use expanded huntingtin in yeast to track how it influences the aggregation of other cellular factors.
"This helps us understand some of the toxic effects that Huntington's has on our cells," said Newby. "We can now measure directly each protein and determine if it's being interfered with."
They also used the technology to investigate two yeast prions. They discovered genetic mutations in the prion gene capable of curing each of the prions, but were surprised to find that the mutations for the two prions were in totally separate classes.
"This tells us that the same mutations, or even the same kinds of mutations, might not be influential in affecting the aggregation of different proteins," said Newby. "It suggests we might need to screen every protein individually if we want to find out how we can cause it to aggregate or stop aggregating."
The team also applied their screening system to study a phenomenon known as prion-switching, in which populations of yeast switch to different prion states in response to an environmental stressor. The phenomenon is thought to be an adaptive mechanism that increases the population's chances of survival in the context of a rapidly-changing environment.
The screening system is currently based on yeast, but Newby said the team plans to adapt it for use with cultures of human cells in the coming months. "All of the individual components that we used, each of the synthetic proteins, should work equally well in human cells as they do in yeast," he said. "We think we can move this into human cells and track human disease proteins in their native contexts."


















