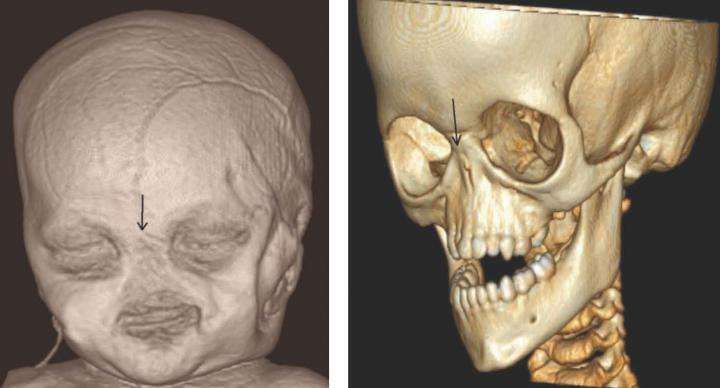
Team identifies gene mutations behind lack of a nose
Researchers from Massachusetts General Hospital (MGH) led a large, international research team that has identified gene mutations associated with a rare congenital condition involving the absence of a nose and often accompanied by defects involving the eye and reproductive systems. Surprisingly, mutations in the same gene, SMCHD1, have previously been associated with a form of muscular dystrophy. The findings are described in a report published in Nature Genetics.
"Fascinating genetic studies had been done on SMCHD1 that linked the gene to FSHD2, a rare muscular dystrophy involving the interaction of multiple genetic sites, but it had never been connected to craniofacial abnormalities," says Michael Talkowski, PhD, of the MGH Center for Human Genetic Research, co-senior author of the Nature Genetics paper. "Our finding from statistical analyses of all genes in the genome that SMCHD1 was the only plausible site of causal variants for arhinia - lack of a nose - was frankly shocking, since prior to our study no patients had ever been reported with both conditions."
The study began with a collaboration among the two lead authors - Harrison Brand, PhD, a research fellow in Talkowski's lab, who sequenced and analyzed the genomes of patients with arhinia, and Natalie Shaw, MD, then with the MGH Reproductive Endocrine Unit and now at the National Institute for Environmental Health Science, who was investigating the lack of reproductive development in a few patients with arhinia. They reached out to clinicians worldwide to identify patients with arhinia - before this study only 80 cases had been reported during the past century - and gathered samples for genetic sequencing.
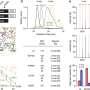
The MGH investigators screened the genomes of 40 individuals with arhinia and 55 family members, from a total of 38 families, revealing rare single-nucleotide mutations within the SMCHD1 gene in 84 percent of affected individuals. SMCHD1 codes for a protein that regulates the expression of other genes and had previously been shown to contribute to FSHD2 (fascioscapulohumeral muscular dystrophy 2), a condition characterized by muscle weakness affecting the face, shoulders and upper arms.
Prior to this study, no individual had been reported with both ahrinia and FSHD2. But evidence from two of the families in the current study suggests that such individuals do exist. As an example, in one family a child with ahrinia had inherited an SMCHD1 mutation from his father, who did not have arhinia but was later found to have symptoms suggestive of FSHD2.
This initial gene discovery led to a series of complementary experiments by collaborators seeking to understand how these SMCHD1 mutations may cause arhinia. Investigators at Duke University, led by Erica Davis, PhD, a co-senior author of the report, blocked the gene's expression in zebrafish, which resulted in abnormal facial cartilage, smaller eyes and structural abnormalities of neurons involved in the development of reproductive organs. A team led by co-senior author David Fitzpatrick, BMBS, at the University of Edinburgh did not find any effects of SMCHD1 mutations in mice, and Talkowski notes that this may reflect the involvement of several mutations in arhinia - similar to the way SMCHD1 mutations contribute to FSHD2 - and that many factors can alter how the conditions are manifested. To explore this question, Peter Jones, PhD, and his team - then at the University of Massachusetts and now at University of Nevada, Reno - investigated in arhinia patients patterns of methylation - which can suppress transcription of a DNA segment - at a region of the genome known to be altered in FSHD2, revealing that the arhinia patients often had identical methylation changes in the same region.
"Among families having several members with SMCHD1 mutations, we observed some who did not have complete arhinia but had an abnormally small nose or lacked a sense of smell, as well as the one individual that had symptoms of FSHD2 but no craniofacial abnormalities, indicating this is not a simple genetic model," says Talkowski. "We still have a lot of questions to answer, including how these mutations interfere with craniofacial and reproductive development and why the same mutations can have such different effects in different individuals. But our findings do indicate that patients with arhinia may be at risk and should be evaluated for the potential to develop FSHD2 and that individuals with SMCHD1 mutations may be at risk for having a child with arhinia."
An associate professor of Neurology at Harvard Medical School, Talkowski adds, "Individuals in 10 countries - clinicians, investigators, patients and family members - helped us assemble the largest group of arhinia patients every studied, encompassing 24 percent of the previously reported 80 individuals and 21 newly identified patients. And our collaborators dedicated the complementary expertise of their laboratories to provide an array of functional studies for this large consortium effort. Without their collaboration, our findings would not have been possible."
More information: Natalie D Shaw et al, SMCHD1 mutations associated with a rare muscular dystrophy can also cause isolated arhinia and Bosma arhinia microphthalmia syndrome, Nature Genetics (2017). DOI: 10.1038/ng.3743