Newborn screening tests approved
(HealthDay)—A set of screening tests designed to detect four rare metabolic disorders in newborns has been approved by the U.S. Food and Drug Administration.
The "Seeker" system of diagnostics is designed to screen for Mucopolysaccharidosis Type 1, Pompe, Gaucher and Fabry. These are the first tests approved to screen for the inherited disorders, which affect proteins that normally eliminate harmful substances from the body, the FDA said in a news release.
The disorders occur in as few as 1 in 185,000 births, or as many as 1 in 1,500 births, depending on the disorder, the agency said. The conditions, collectively called Lysosomal Storage Disorders, could lead to organ damage and death if not treated in a timely way, the FDA added.
Some states now require screening of these disorders, the agency said, including Arizona, Illinois, Kentucky, Michigan, Missouri, New Jersey, New Mexico, New York, Ohio, Pennsylvania and Tennessee.
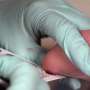
The newly approved tests require blood samples collected from the prick of a newborn's heel within 48 hours of birth. The FDA said it reviewed data from a clinical study of more than 154,000 infants in Missouri. The system identified at least one of the four disorders in 73 of the screened newborns, the agency said.
The tests are produced by Baebies Inc., based in Durham, N.C.
More information: Visit the FDA to learn more.
Copyright © 2017 HealthDay. All rights reserved.