Scientists create scorecard index for heart-damaging chemo drugs
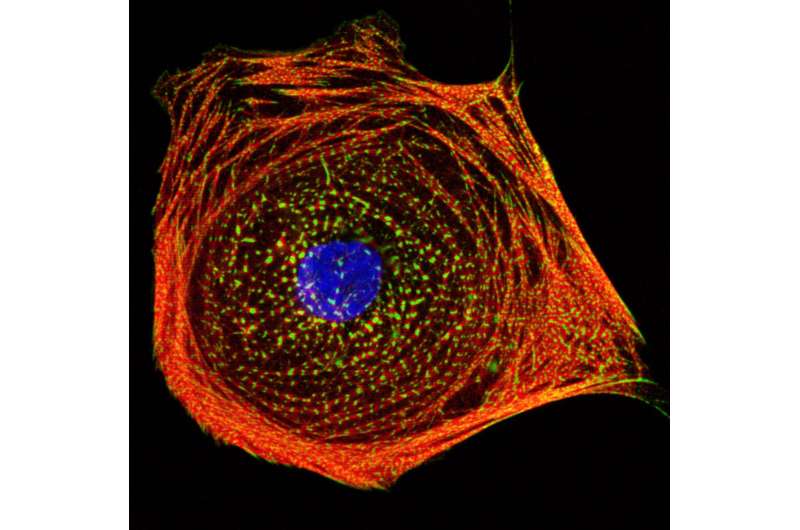
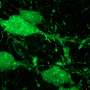
Researchers at the Stanford University School of Medicine used heart muscle cells made from stem cells to rank commonly used chemotherapy drugs based on their likelihood of causing lasting heart damage in patients.
Drugs known as tyrosine kinase inhibitors can be an effective treatment for many types of cancers, but they also have severe and sometimes fatal side effects. Using lab-grown heart cells, Stanford researchers were able to assess the drugs' various effects on heart muscle cells, including whether the cells survived, were able to beat rhythmically and effectively, responded appropriately to electrophysiological signals and communicated with one another.
The researchers found that their assay can accurately identify those tyrosine kinase inhibitors already known to be the most dangerous in patients. In the future, they believe their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
"This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients," said Joseph Wu, MD, PhD, director of the Stanford Cardiovascular Institute and a professor of cardiovascular medicine and of radiology. "It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects."
A paper describing the research will be published Feb. 15 in Science Translational Medicine. Wu, who holds the Simon H. Stertzer Professorship, is the senior author. Former graduate student Arun Sharma, PhD, is the lead author.
'Multiple measurements'
"We used multiple measurements to accurately predict which of the tyrosine kinase inhibitors were the most cardiotoxic," said Sharma. "The drugs with the lowest safety indices in our study were also those identified by the Food and Drug Administration as the most cardiotoxic to patients. Other drugs that are not as cardiotoxic performed much better in our assays."
Validating the researchers' cardiac-safety test on drugs with extensive clinical track records is necessary before the assay can be used to predict with confidence the likely clinical outcomes of drugs still under development.
Sharma, Wu and their colleagues created heart muscle cells called cardiomyocytes from induced pluripotent stem cells, or iPS cells, from 11 healthy people and two people with kidney cancer. They grew the lab-made cardiomyocytes in a dish and tested the effects of 21 commonly used tyrosine kinase inhibitors on the cells.
They found that treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction. The researchers used these and other measurements to develop a cardiac safety index for each drug.
They found that those drugs known to be particularly dangerous to heart function, such as nilotinib, which is approved for the treatment of chronic myelogenous leukemia, and vandetanib, which is approved for the treatment of some types of thyroid cancer, also had the lowest safety indices based on the assay; conversely, those known to be better tolerated by patients ranked higher on their safety index. Prescribing information for both nilotinib and vandetanib contains warnings from the FDA about the drugs' potential cardiotoxicity.
An activity increase in an insulin responsive pathway
Six of the 21 tyrosine kinase inhibitors tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic. Three of these six are known to inhibit the same two signaling pathways: VEGFR2 and PDGFR. The researchers noticed that cells treated with these three drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulinlike growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further. They found that exposing the cells to insulin or IGF1 made it less likely they would die due to tyrosine kinase inhibitors blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these chemotherapies.
The current study mirrors another by Wu's lab that was published in April 2016 in Nature Medicine. That research focused on the toxic effect of a chemotherapy drug called doxorubicin on iPS cell-derived cardiomyocytes. Doxorubicin, which indiscriminately kills any replicating cells, is increasingly being replaced by more targeted, cancer-specific therapies such as the tyrosine kinase inhibitors tested in the current study.
"The switch from doxorubicin is a result of the paradigm shift in cancer treatment to personalized, precise treatment as emphasized by President Obama's 2015 Precision Medicine Initiative," said Wu. "Moving even further, we're discovering that many tyrosine kinase inhibitors are themselves significantly cardiotoxic, and some have been withdrawn from the market. There is a critical need for a way to 'safety test' all drugs earlier in development before they are administered to patients. Our drug safety index is a step in that direction."
More information: "High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells," Science Translational Medicine, stm.sciencemag.org/lookup/doi/ … scitranslmed.aaf2584