New treatment option for difficult-to-treat rheumatoid arthritis patients
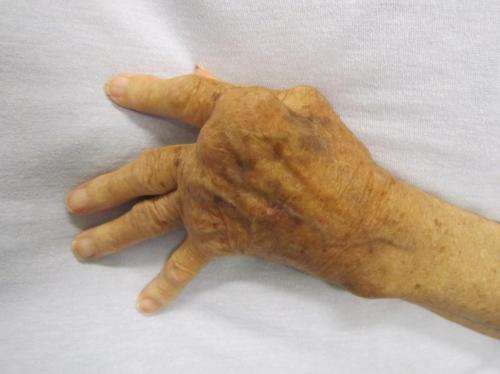
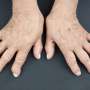
Between 3 and 5 percent of the population suffers from a form of inflammatory rheumatism. It affects approximately 250,000 to 400,000 people in Austria. Rheumatoid arthritis is one of the most common and also the most dangerous forms of this inflammatory rheumatic disease. Around 30 percent of patients achieve remission, described as successful control of symptoms, after just one or two years. However, despite frequent changes in treatment, many patients endure the active form of the disease on an ongoing basis. A multicentre, multinational study headed up by rheumatologist Daniel Aletaha of MedUni Vienna as principal investigator has now shown that a new drug (sirukumab) is a promising treatment option for these "refractory" patients. The study has now been published in The Lancet.
Today, doctors change treatments for rheumatoid arthritis very quickly if one treatment fails to effect any significant improvement in a patient. This means that many patients can be helped very quickly. On the other hand, there are patients who do not show any significant improvement even after the second or third biologic drug treatment – typically with tumour necrosis factor (TNF) inhibitors. TNF is involved in systemic inflammation. The new drug now offers a promising option for such patients.
This is the result of one of the largest multicentre, international studies ever conducted into difficult-to-treat rheumatoid arthritis. It focuses on a new mechanism of action, an interleukin-6 cytokine blockade. In this treatment, the monoclonal antibody sirukumab directly inhibits the messenger substance IL-6, which, like TNF, is responsible for inflammatory processes in the joints.
"We were able to demonstrate this in one of the largest study populations to date, with around 900 patients in 35 countries. Despite having previously received treatments with biologic drugs, these patients still had a persistently active disease. Treatment options had been practically exhausted for many of these patients. However, even in this group of patients, treatment with sirukumab brought about a significant reduction in the inflammatory action of the disease," says Daniel Aletaha of MedUni Vienna's Department of Medicine III.
The efficacy and safety of sirukumab were tested in two different dosages (injections of 50 mg every four weeks or 100 mg every two weeks). The 100 mg dosage proved to be slightly more effective. "These results are very significant in the case of a progressive, inflammatory, musculoskeletal disease such as rheumatoid arthritis, especially for those patients who are resistant to treatment," says the MedUni Vienna expert. The drug could be approved very soon.
At the same time, the new findings with sirukumab could lead to new efficacy studies being instigated for other inflammatory diseases, such as other forms of arthritis or other inflammatory diseases, e.g. vasculitis. There may also be other indications in areas other than rheumatology.
More information: Daniel Aletaha et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study, The Lancet (2017). DOI: 10.1016/S0140-6736(17)30401-4