April 27, 2017 report
Using a smartphone and engineered cells to control diabetes in mice

(Medical Xpress)—A team of researchers affiliated with several institutions in China has developed a way to combine a smartphone with a glucose monitor and engineered cells to automatically control insulin levels in test mice. In their paper published in the journal Science Translational Medicine the team describes their technique and how well it worked in the mice. Mark Gomelsk with the University of Wyoming offers a Focus piece in the same issue highlighting the work done by the team.
Millions of people around the world have diabetes, and many of them have to inject insulin to keep their glucose levels in check. This generally involves taking periodic blood samples for testing to determine whether and when shots are needed. While this approach works, it is not optimal, because testing and injecting can be inconvenient and because unanticipated glucose spikes or drops can occur due to a variety of factors such as types or amount of food eaten or engaging in exercise. In this new effort, the researchers report the development of a closed loop system that offers a way to produce insulin in the body and distribute it on an as-needed basis.
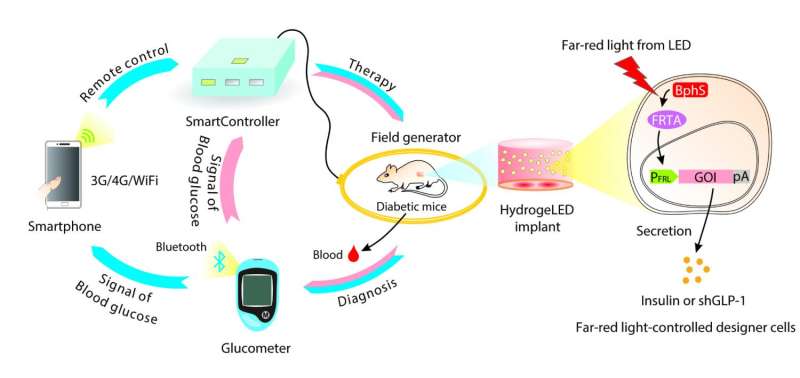
The new system by the team in China involved engineering insulin-producing cells to do their work when illuminated with infrared light. The researchers placed the cells in an insulated sheath that also contained red LED lights—then, they placed the sheath under the skin of test mice. They controlled the lights remotely via smartphone app, sending signals to a control box containing a coil that activated the lights. The smartphone received data from an embedded blood glucose meter. The result was a closed-loop system in which the glucose meter automatically conducted glucose testing on a periodic basis. The smartphone app analyzed the data to determine when and how much insulin needed to be produced. It then sent a signal to the control box, activating the LED lights, causing the cells to produce and release insulin into the bodies of the mice.

The researchers tested their system with mice over a period of several weeks and report that it successfully maintained insulin levels. They note that their work represents an important step toward creating such devices for use in humans.
More information: Jiawei Shao et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice, Science Translational Medicine (2017). DOI: 10.1126/scitranslmed.aal2298
Abstract
With the increasingly dominant role of smartphones in our lives, mobile health care systems integrating advanced point-of-care technologies to manage chronic diseases are gaining attention. Using a multidisciplinary design principle coupling electrical engineering, software development, and synthetic biology, we have engineered a technological infrastructure enabling the smartphone-assisted semiautomatic treatment of diabetes in mice. A custom-designed home server SmartController was programmed to process wireless signals, enabling a smartphone to regulate hormone production by optically engineered cells implanted in diabetic mice via a far-red light (FRL)–responsive optogenetic interface. To develop this wireless controller network, we designed and implanted hydrogel capsules carrying both engineered cells and wirelessly powered FRL LEDs (light-emitting diodes). In vivo production of a short variant of human glucagon-like peptide 1 (shGLP-1) or mouse insulin by the engineered cells in the hydrogel could be remotely controlled by smartphone programs or a custom-engineered Bluetooth-active glucometer in a semiautomatic, glucose-dependent manner. By combining electronic device–generated digital signals with optogenetically engineered cells, this study provides a step toward translating cell-based therapies into the clinic.
© 2017 Medical Xpress