Antibody that neutralizes sMIC boosts CTLA4 immunotherapy response and reduces colitis

Co-administering a monoclonal antibody that neutralizes tumor-released soluble MHC I chain-related molecule (sMIC) improves anti-CTLA4 antibody therapy effectiveness and reduces treatment-related colitis, report Medical University of South Carolina (MUSC) investigators in an article published online May 17, 2017 by Science Advances.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA4) can be thought of as an 'off switch' or 'immune checkpoint' for effector T cells that are activated to fight cancer tumors. Researchers have developed a therapy to block CTLA4, specifically, anti-CTLA4 antibody immunotherapy, to help sustain T-cell activation and improve patient survival.
Unfortunately, while anti-CTLA4 therapy is highly effective in animals, response rates in humans vary widely and serious adverse events such as colitis (gastrointestinal inflammation) are common. For example, Ipilimumab, an FDA-approved anti-CTLA4 antibody therapy for advanced melanoma, is highly effective in controlling tumors in mice but has a response rate of only fifteen percent in humans. Clinical investigators have tried to improve the efficacy and safety of anti-CTLA4 therapy by combining it with other agents but response rates and toxicity remain suboptimal.
A team of MUSC researchers led by Jennifer Wu, Ph.D., professor of Microbiology and Immunology, suspected that response disparities between humans and animals may be due to differences in immune modulators that human tumors express.
"When we use animals to study therapies for humans we often neglect certain human-specific biological pathways simply because they don't exist in the animal," explains Wu. "MIC is one of those molecules that is expressed in human tumors but is absent in mice. We knew that the soluble form, sMIC, is highly immunosuppressive in humans and we knew it was important, but we had no way to study it. We had to create a new model."
Their work to unravel these molecular-level differences has now paid off, with the discovery of a new combination therapy that dramatically improves CTLA4 therapy effectiveness and avoids therapy-induced colitis.
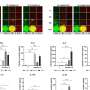
The team developed a clinically relevant, MIC-transgenic spontaneous mouse tumor model that closely resembled the onco-immune dynamics seen in human cancers. Using this model, they investigated how tumor-derived, human sMIC affected anti-CTLA4 therapy. They found that high blood levels of sMIC not only reduced the antitumor efficacy of anti-CTLA4 therapy but also directly evoked colitis.
"It was a total surprise, and we were a little nervous about the results," says Wu. "So, we repeated the test using multiple models and antibodies and different batches of animals to make sure it was reproducible and that what we were seeing was real. Sometimes knowledge itself gets in the way. Because there are certain accepted beliefs that make you think what you're seeing can't be true-that it's impossible. But when multiple experiments are all coming up with the same results, what can you say? That's when we have to let go and come to a new understanding."
Next, the team co-administered an antibody called B10G5 that neutralizes sMIC, alongside anti-CTLA4 therapy. The new combination therapy not only remarkably improved anti-CTLA4 immunotherapy effectiveness, but also alleviated therapy-induced colitis.
"We've been studying B10G5 for a while and published a paper in Clinical Cancer Research in 2015 demonstrating that using B10G5 to target sMIC can alleviate tumor-induced immune suppression and also has a huge immune-stimulating ability," explains Wu. "So those results led us to think it would be a good strategy to combine B10G5 with antibodies that target immune checkpoint molecules, the 'off-switch' of an ongoing immune response."
Finally, inspired by a case study published in 2006, the team decided to try to validate its current findings in humans. The original case reported a melanoma patient who developed anti-MIC autoantibody during anti-CTLA4 therapy and had a superior therapeutic response. So, they contacted a long-time collaborator at Oregon Health and Science University and Knights Cancer Center to obtain plasma samples collected during a clinical trial they were conducting in metastatic prostate cancer patients.
Wu's team looked for anti-sMIC autoantibodies in samples from ten patients receiving anti-CTLA4 therapy with Ipilimumab. One sample showed high levels of anti-MIC autoantibody. When the team followed up with their colleague, it turned out that this particular cancer patient not only demonstrated a remarkable therapeutic response (his prostate-specific antigen fell from 191 to 4.6 ng/ml after eight treatment cycles) but also did not develop autoimmune colitis.
That case reinforced the team's preclinical in vivo findings that coadministering the sMIC-neutralizing antibody (B10G5; CanCure, LLC) enhances anti-CTLA4 therapy and deters colitis. Overall, these results indicate a new, powerful combination immunotherapy for cancer. They also suggest that pre-screening serum sMIC levels might help clinicians to identify patients who are most likely to have a positive therapeutic response.
"I hope that our findings will inspire investigators to revisit other cancer immunotherapies that were successful in animals but presented no efficacy in humans," says Wu. "Anti-CTLA4 therapy is approved for melanoma and also is still in trials for other cancers. Maybe our study will inspire clinical investigators to think about screening their patients to identify who will be a better responder versus a poor responder to anti-CTLA4 therapy."
More information: "Antibody-mediated neutralization of soluble MIC significantly enhances CTLA4 blockade therapy" Science Advances (2017). advances.sciencemag.org/content/3/5/e1602133