A new insight into Parkinson's disease protein
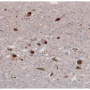
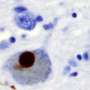
Abnormal clumps of certain proteins in the brain are a prominent feature of Parkinson's and other neurodegenerative diseases, but the role those same proteins might play in the normal brain has been unknown.
Now, new research by UC San Francisco neuroscientist Robert Edwards, MD, has uncovered the role of one such protein, known as alpha-synuclein, which has long been implicated in Parkinson's disease. Understanding how alpha-synuclein normally acts, he said, can provide direct clues to how it might fail, and may point the way to preventing or managing diseases including Parkinson's.
This finding is the first identification of a normal role for a signature protein central to neurodegenerative disease.
"The aggregation of alpha-synuclein in neurons is a hallmark of Parkinson's," Edwards said. "But we wanted to search for earlier events that might trigger the degenerative process."
Alpha-Synuclein in Healthy Nerve Cells
In healthy nerve cells, alpha-synuclein can be detected at synapses, the sites where bubble-like vesicles fuse with a neuron's surface to release the signaling molecule dopamine, which is known to be depleted in Parkinson's, said Edwards, who was elected to the National Academy of Sciences in May 2017. "A disturbance in this membrane-fusing process would disrupt the normal communication between neurons required for essentially all brain activity."
As reported in Nature Neuroscience, in a series of imaging experiments with neurons and other cells from mice, Edwards's research team explored exactly how alpha-synuclein takes part in this crucial interaction.
The protein actually plays two roles, they found. When present in abnormally high amounts, as it is in Parkinson's, it can inhibit the fusion of vesicles with membranes, which is necessary for neurotransmitter release. But in normal amounts, it has a surprisingly different effect: if neurotransmitters are already being released, alpha-synuclein helps speed the process.
The question remains how one protein can have both negative and positive roles in transmitter release, and how the former might contribute to Parkinson's disease.
A smart guess might be that Parkinson's arises from an abnormal form of alpha-synuclein inhibiting dopamine release. But Edwards, the John and Helen Cahill Family Endowed Chair in Parkinson's Disease Research, found strong evidence for a less intuitive explanation.
Mutations, Unknown Mechanisms
Several mutations in the synuclein gene are known to cause rare forms of Parkinson's that run in families. Edwards showed that they do so not by boosting synuclein's inhibitory effect, but by disrupting its normal ability to aid neurotransmitter release once it has started. "So when the negative effect is intact, but the positive role is blocked, the net result disrupts neurotransmitter release," he surmises. "This could lead to the impairment in dopamine release, and ultimately to cell death."
The vast majority of people with Parkinson's don't inherit it through mutations, but acquire it by unknown mechanisms. Edwards's research has convinced him that the more common form of the disease arises from a defect in alpha-synuclein's natural ability to speed neurotransmitter release.
He hasn't pinpointed a potential cause for this defect in most people with the disease, but thinks one suspect is phosphorylation, a cellular process that regulates normal protein function.
"By understanding the normal function of alpha-synuclein, and in particular its regulation, we're starting to understand how anyone can acquire Parkinson's disease," Edwards said. "We hope this can help develop therapy that targets the underlying degenerative process."
More information: Todd Logan et al. α-Synuclein promotes dilation of the exocytotic fusion pore, Nature Neuroscience (2017). DOI: 10.1038/nn.4529