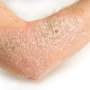
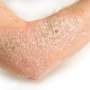
ABP 501, adalimumab biosimilar, safe and effective, for psoriasis
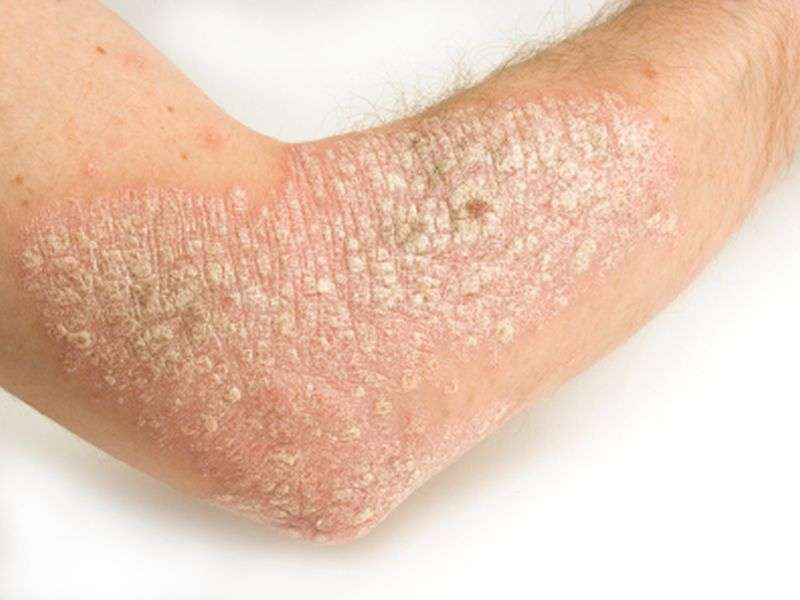
(HealthDay)—The biosimilar ABP 501 has similar clinical efficacy and safety to adalimumab for the treatment of moderate-to-severe plaque psoriasis, according to a study published online July 28 in the British Journal of Dermatology.
Kim Papp, M.D., Ph.D., from Clinical Research and Probity Medical Research in Waterloo, Canada, and colleagues randomized 308 patients (1:1) to receive ABP 501 or adalimumab (40 mg) every two weeks for 16 weeks. At week 16, patients with at least 50 percent improvement from baseline in psoriasis area-and-severity index score (PASI) were eligible to continue to Week 52 (152 patients), whereas adalimumab patients were re-randomized (1:1) to continue adalimumab (79 patients) or undergo a single transition to ABP 501 (77 patients).
The researchers found that PASI percent improvements were similar across groups for weeks 16, 32, and 50 (range, 85.8 to 88.2 percent), with no significant differences across groups in percentages of PASI 50, 75, 90, and 100 responders. Similarly, changes in percent body surface area affected were similar across groups and timepoints. There were no new safety issues reported, with adverse events similar between the groups.
"ABP 501 and adalimumab have similar clinical efficacy, safety, and immunogenicity profiles over 52 weeks, including after single transition, in this patient population," the authors write.
Several authors disclosed financial ties to pharmaceutical companies, including Amgen, which manufactures ABP 501 and funded the study.
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2017 HealthDay. All rights reserved.