New treatment approved for acute myeloid leukemia
(HealthDay)—The combination chemotherapy drug Vyxeos (daunorubicin and cytarabine) has been approved by the U.S. Food and Drug Administration as the first treatment for certain high-risk types of acute myeloid leukemia (AML).
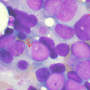
AML is an aggressive blood cancer that forms in the bone marrow.
"Vyxeos combines two commonly used chemotherapies into a single formulation that may help some patients live longer than if they were to receive the two therapies separately," said Dr. Richard Pazdur, director of the FDA's Oncology Center of Excellence.
In a news release Thursday, the agency said more than 21,000 people will be diagnosed this year with AML, and more than 10,000 will die from it, according to projections from the U.S. National Cancer Institute.
The new therapy is sanctioned for high-risk forms of newly-diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). People with either disease have a very low life expectancy, the FDA said.
Vyxeos was evaluated in clinical trials involving 309 people with either form of AML. Those given Vyxeos lived an average of 9.6 months, compared with 5.9 months among those who took an inactive placebo.
The therapy's most common side effects included bleeding, fever, low white blood-cell count, rash, tissue swelling and nausea. Some users had episodes of serious allergic-like hypersensitivity reactions or dangerous bleeding, the agency said.
Women who are pregnant or breast-feeding shouldn't take Vyxeos, the FDA added.
Approval of the drug was granted to the Irish firm Jazz Pharmaceuticals.
More information: To learn more about this approval, visit the FDA.
Copyright © 2017 HealthDay. All rights reserved.