Scientists are diving deep into the structure and function of ion channels to inform new therapies
A growing cohort of talented Northwestern Medicine scientists is working to unlock the secrets of ion channels and discover how these tiny molecular machines contribute to an array of diseases, from brain tumors and epilepsy to kidney disease and devastating immune deficiencies.
This group of investigators, including seasoned faculty like Alfred George Jr., MD, Magerstadt Professor and chair of Pharmacology, and newcomers like Paul DeCaen, PhD, assistant professor in the same department, are not only fundamentally altering understanding of disorders, they're also revealing how existing treatments work and pointing to potential new treatment strategies.
"All of this expertise provides fertile ground for making new discoveries," says DeCaen, a former Howard Hughes and Harvard University fellow who joined Northwestern in October 2016. "And a world-class hospital here gives us access to the medical perspective on ion channel-linked diseases."
The shape of things
Ion channels are a class of proteins that control the flow of ions such as calcium, sodium or potassium across the membranes of cells, DeCaen explains. Maintaining a proper flow of ions is critical to a multitude of bodily functions, from the transmission of messages between brain cells to the beating of the heart.
"It seems like a simple job, but it ends up frequently being problematic," he says.
Mutations in the genes that encode ion channels have been linked to many medical conditions. To understand how these mutations lead to disease, ion channel investigators try to piece together the three-dimensional molecular structures of ion channels.
For example, DeCaen and colleagues from the lab of Erhu Cao, PhD, at the University of Utah took this approach to better understand a gene called polycystic kidney disease 2 (PKD2). Mutations in the gene had been found in patients who develop large cysts in their kidneys that cause organ failure. Scientists knew the gene encoded an ion channel that controls the flow of ions, but did not know which ions. Work from DeCaen's lab pointed to potassium and sodium.
"We now know what ions move through the channel, but no one had any idea of what it looked like in three-dimensional space," DeCaen says. "Since function follows form, we figured that this is an important knowledge gap to fill."
So, the team chilled the protein to a very low temperature and then used a powerful electron microscope to get the first glimpse of the protein's configuration. The results were published in the journal Cell last year.
"Now that we know what the ion channel looks like, we can see how mutations that cause alterations in its structure may cause it to malfunction in the disease state," he says. "We can start to do some pie-in-the-sky thinking about developing small molecules that can affect the ion channel's function."
For example, in polycystic kidney disease it is not clear whether mutations cause the PKD2 channel to be continually open, allowing an unending flow of ions, or if the mutation closes the channel. There might even be a mix of on/off effects depending on the specific mutation. So, DeCaen and colleagues are using electrophysiological techniques to find out. Their results could inform the design of drugs to combat the disease.
DeCaen has also been consulting Northwestern clinicians about complications beyond cysts in patients with polycystic kidney disease. These clinical insights might provide clues on the function of these ion channels throughout the body and potentially suggest treatment strategies.
"In ion channel research, you need a broad range of expertise in medicine," DeCaen explained. "You need a neurologist, a cardiac arrhythmias expert and kidney disease experts. We have that large pool of scientists and clinicians here at Northwestern."
Working with George, and Jennifer Kearney, PhD, associate professor of Pharmacology, DeCaen is also probing the role of ion channels in epilepsy. His lab is recreating the structure of a bacterial version of an epilepsy-linked sodium channel as a first step toward recreating the mammalian version. So far, the work has yielded unexpected clinical benefits.
"This gave us our first glimpse into how anti-epileptic drugs work," DeCaen says. It has also suggested potential antibacterial treatments that would target the channel.
The applications of this line of research go even further: This summer, George and colleagues showed how mutations in a sodium channel called Nav1.9 can lead to a disorder where people are unable to feel pain. The findings, published in The Journal of Clinical Investigation, might have implications for the development of novel therapies for pain.
"Ion channels represent an under-appreciated class of druggable protein targets," says George. "A goal for the Department of Pharmacology has been to place ion channels at the center stage of research efforts to find new drug targets."
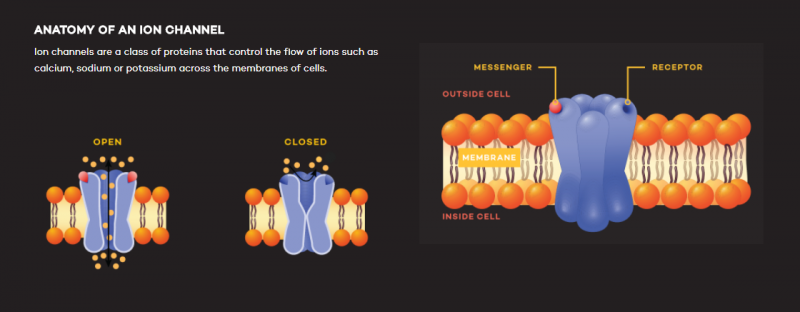
Moving parts
Meanwhile, Murali Prakriya, PhD, associate professor of Pharmacology, focuses on the Ca2+ release-activated Ca2+ (CRAC) channel. Originally described in immune cells, CRAC channels are found in the plasma membranes of most, if not all, human cells. When the channel opens, it allows calcium ions to flow into the cell, signaling functions such as gene expression and cell proliferation. A growing number of diseases are associated with abnormalities in CRAC channel function including immunodeficiencies, muscular dystrophy and neurological diseases such as Alzheimer's disease.
"CRAC calcium channels are widespread and important for many biological processes, from the birth of cells to the death of cells," Prakriya says. "Therefore, dissecting how CRAC channel activity is controlled and regulated in different contexts is of great interest."
His lab is working to understand how CRAC channels operate and contribute to immune host defense mechanisms, the detection of allergens in the lung airways, and brain function.
"If you lose CRAC channel function through mutations, human patients develop devastating immune deficiencies and muscle weakness," he explains. "Children born with these symptoms often die in the first six months of life. The simplest infections are quite dangerous to these children."
In a paper published in Nature Communications early this year, Prakriya worked with Megumi Yamashita, PhD, DDS, research assistant professor of Pharmacology, and Priscilla Yeung, a student in Feinberg's Medical Scientist Training Program, to reveal how the CRAC channel opens and closes. This research identified the molecular structure in the channel that functions as the gate, as well as the movements in the channel pore that open the gate.
First, the scientists used electrophysiology and microscopy techniques to systematically probe the contributions of different regions of the CRAC channel protein to pore opening, identifying an oily amino acid as the channel gate in the process. Then, computer simulations developed by University of Toronto collaborators helped reveal how this amino acid impedes ion conduction.
"In ion channels, the pore is usually filled with water, so one way to close the pore is to present an oily, hydrophobic chemical group in the pore to prevent water and ions from going through—similar to the way that oil and water don't mix. To open the pore, the hydrophobic group swings out of the way allowing the pore to fill with water and ions," Prakriya explains. "The presence of the oily amino acid in the pore creates a closed channel state."
These conclusions have important clinical implications. Some human mutations in the gene encoding the CRAC channel leave the gate open and cause uncontrolled bleeding, neurological problems and muscle weakness because the cells in these individuals have excessive levels of calcium all the time.
"We showed that one of these mutations affected the oiliness of the gate region, thereby chronically filling the pore with water and ions," Prakriya says. "As a consequence, ions were going through when they shouldn't."
Prakriya's lab is currently working to understand the molecular signals that open the hydrophobic gate and to identify small molecules that can interact with the gate to alter the channel's activity. These could correct defects in cell signaling and ameliorate symptoms associated with aberrant CRAC channel activity seen in immune, muscular and neurodegenerative diseases.
Translating discoveries
While investigators like DeCaen and Prakriya focus on molecular-level details, Rintaro Hashizume, MD, PhD, assistant professor of Neurological Surgery and of Biochemistry and Molecular Genetics, is using mouse models of brain tumors to begin to translate basic ion channel discoveries into experimental therapeutics.
Before he joined Northwestern in 2014, Hashizume collaborated with a team of ion channel investigators at the University of California, San Francisco, who figured out that medulloblastoma, a cancerous pediatric brain tumor, was enriched with Ether-a-go-go 2 (EAG2) potassium ion channels.
The EAG2 channel helps regulate the cell cycle and volume of cells, so the investigators searched for a drug that could inhibit it. They found that thioridazine, used to treat schizophrenia, did the trick. Hashizume gave the drug to mice with human medulloblastoma and showed that it stopped tumor growth and, more importantly, prevented metastasis, which occurs when the tumor spreads to other parts of the body, decreasing patient survival rates. The findings were published in Nature Neuroscience.
"That's an important therapeutic advantage of the potassium ion channel blocker—if the tumor doesn't metastasize you can focus on the management of the original tumor," he says.
Hashizume has since launched a pediatric tumor research collaboration with George. Using cells derived from a Northwestern pediatric patient with a brain tumor, Hashizume created a mouse model that will allow the team to probe how the mutation affects ion channel function and test treatments that might correct the problem.
While this type of fundamental science and early translational research may not be fodder for a Hollywood blockbuster, DeCaen notes that it's the type of research that may lead to big clinical gains in the long run.
"When you get down to the nitty gritty of how a protein works, that is where we really make true breakthroughs," DeCaen says. "Sometimes it takes a lot of sweat and hard work to try to understand the minutiae. But these types of studies have yielded a significant impact towards the development of therapeutic drugs that target ion channels."
More information: Megumi Yamashita et al. STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate, Nature Communications (2017). DOI: 10.1038/ncomms14512