December 15, 2017 report
Testing shows differences in efficacy of Zika vaccines after one year
(Medical Xpress)—A large team of researchers with members from Harvard Medical School, Walter Reed Army Institute of Research, Bioqual Inc. and MIT has found that the efficacy of the three types of Zika vaccines currently undergoing clinical trials varied widely in monkeys after one year. In their paper published in Science Translational Medicine, the group describes testing all three vaccines in rhesus monkeys and what they found.
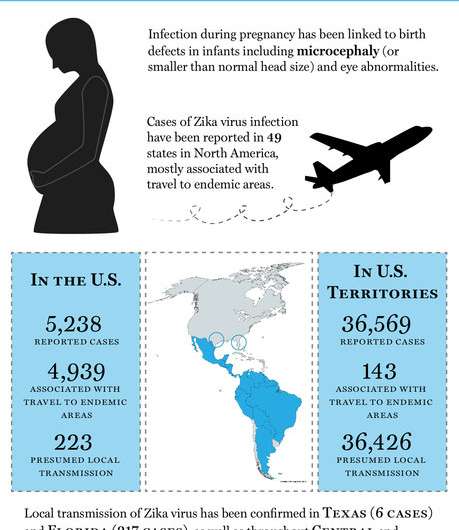
The Zika virus has been widely reported in the press in recent years due to a surge in infections in South America and, and the heartbreaking consequences—babies with birth defects born to infected mothers. Fortunately, progress has been made in developing a vaccine to protect humans from the virus. To date, there are three leading candidates. One is based on injections of purified/inactive Zika virus samples; another is adenovirus vector-based, which means it is carried by one of the family of viruses that cause the common cold—and the third is DNA-based.
The three vaccines are currently at different stages of testing, though it is hoped that at least one of them will be available for commercial use very soon. One aspect of Zika vaccination that is not known, the researchers note, is how well they work after a length of time has passed. To find this answer for themselves, the researchers tested all three in monkeys over the course of a whole year.
The team reports that the DNA vaccine was no longer effective after a year had passed, suggesting it likely will not be the vaccine selected for use in humans—the inactive viral sample vaccines were better at approximately 75 percent effectiveness, but it was the adenovirus-based vaccine that showed the best performance offering monkeys 100 percent immunity against the virus a year after being given a single injection.
The researchers acknowledge that it is not clear if the three vaccines would offer the same results in humans, but note that testing in humans is going to be more difficult, because transmission rates are currently low, making it difficult to find patients to include in the trials. They suggest that studies such as theirs should offer some assistance in the path towards approval of the eventual vaccine.
More information: Peter Abbink et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys, Science Translational Medicine (2017). DOI: 10.1126/scitranslmed.aao4163
Abstract
An effective Zika virus (ZIKV) vaccine will require long-term durable protection. Several ZIKV vaccine candidates have demonstrated protective efficacy in nonhuman primates, but these studies have typically involved ZIKV challenge shortly after vaccination at peak immunity. We show that a single immunization with an adenovirus vector–based vaccine, as well as two immunizations with a purified inactivated virus vaccine, afforded robust protection against ZIKV challenge in rhesus monkeys at 1 year after vaccination. In contrast, two immunizations with an optimized DNA vaccine, which provided complete protection at peak immunity, resulted in reduced protective efficacy at 1 year that was associated with declining neutralizing antibody titers to subprotective levels. These data define a microneutralization log titer of 2.0 to 2.1 as the threshold required for durable protection against ZIKV challenge in this model. Moreover, our findings demonstrate that protection against ZIKV challenge in rhesus monkeys is possible for at least 1 year with a single-shot vaccine.
© 2017 Medical Xpress