Breakthrough cancer therapy raises tough questions about drug costs, value
Imagine a drug that, a month after a single intravenous dose, wipes out all evidence of leukemia in 80 percent of deathly ill children who receive it.
Now, imagine that the drug takes a month to make from each patient's own immune cells, that many children die waiting, that it causes terrible temporary side effects, that it costs $475,000, and that the accompanying medical care adds vastly to that price tag.
Finally, imagine that a year later, the blood cancer has roared back in half of the children who had astounding remissions.
This isn't fantasy. This is the profile of Novartis' Kymriah, the first-of-its-kind CAR T-cell therapy. No wonder it has inspired awe, as well as deep concerns, that the benefits have been overstated and overpriced.
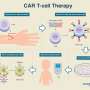
Novartis partnered with the University of Pennsylvania and Children's Hospital of Philadelphia (CHOP) after researchers at those centers pioneered the technology to genetically engineer each patient's immune T cells to attack blood cancer cells. On Feb. 1, the New England Journal of Medicine published updated results from the global clinical trial of 95 youngsters that led to the U.S. Food and Drug Administration's August approval of Kymriah to treat recurrent acute lymphoblastic leukemia.
Reactions to the latest results reflect the scientific, economic, and emotional complexity of the breakthrough.
Vinay Prasad, an oncologist and drug development researcher at Oregon Health and Science University in Portland, said the update confirms his skeptical view of Kymriah's value. He faulted the researchers for inflating their one-year survival rate to 50 percent by excluding kids who gave their T cells but never got treated because of manufacturing problems or because they died before they could get the therapy. By including those children—a standard statistical practice to minimize bias—Prasad calculated the one-year survival rate is 40 percent.
"It's just a cheap way to increase your success rate that does not help patients navigate the decision of 'how likely am I to benefit?'" Prasad said. "The math shows most patients who qualify for this do not benefit. And many other patients will not qualify. So CAR-T is good. It's better than what we've done before, but not great."
John P. Leonard, an oncologist and lymphoma specialist at Weill Cornell Medical College in New York City, agreed that the survival data should reflect the fact that some patients who undergo T cell collection don't get the therapy.
"When you leave out people who didn't get the drug, it doesn't get counted against the drug," Leonard said. Including them "puts it in a little less rosy light. I think it would be valuable to present the data both ways."
Dana Lee of Ocean Township, N.J., looks at the statistics "a little differently," having seen the ravages of chemotherapy, radiation, and bone marrow transplantation on children like her 14-year-old daughter, Tori.
Almost five years ago, Tori became the 10th child to get the T-cell therapy in a pilot study at CHOP. She suffered the characteristic reaction—severe but treatable immune overstimulation, involving high fever, low blood pressure, muscle pain, neurologic symptoms.
She's now an eighth-grade honor roll student who loves basketball—and dislikes getting attention for accidentally being a medical trailblazer.
"One hundred percent of those children were not going to make it," Lee said. "All options were gone. So it gave hope to parents and children who had no hope."
About 6,000 cases of pediatric lymphoblastic leukemia are diagnosed each year in the U.S. and in Europe, where Kymriah approval is imminent. Standard therapies cure 85 percent. For those that repeatedly relapse, the prognosis is grim.
David Mitchell, who has an incurable blood cancer called multiple myeloma, understands the power of desperation, and is thrilled by the advent of CAR T-cell therapies that might one day help him.
But as the founder of the advocacy organization Patients for Affordable Drugs, Mitchell says Kymriah developed with government subsidies—is overpriced by "hundreds of thousands of dollars." That sets a dangerous precedent, he said, given that dozens of CAR T drugs are in the pipeline. (Two pricey gene therapies were approved after Kymriah: Kite Pharma's $375,000 Yescarta for a type of lymphoma, and Philadelphia-based Spark Therapeutics' $850,000 Luxturna for an inherited form of blindness.)
"The key question is not: What's it worth to save a child's life?" Mitchell said. "If that was the question, then the polio (vaccine) they gave me when I was 6 years old would have cost a million dollars. The right question is: What is the price that will maximize accessibility and affordability, while maintaining a robust R&D pipeline?"
In contrast, another organization that assesses drug effectiveness and value, the Institute for Clinical and Economic Review, concludes that Kymriah is cost-effective: "On average, using Kymriah in a pediatric population instead of traditional chemotherapy increases health care costs by approximately $400,000, but extends life by eight years."
Anticipating controversy over the price, Novartis announced that it wouldn't charge for Kymriah if the patient doesn't respond within a month of treatment. (Penn and some of the key scientists stand to benefit financially from the Kymriah partnership, according to the university.) But critics say that deal is rigged, since 80 percent of patients go into remission, even though at least half later relapse.
Controversy has not dampened demand for Kymriah. Stephan Grupp, the CHOP oncologist who has overseen clinical testing of the drug, traveled widely to set up the global trial, conducted at 25 specialized medical centers in North America, Europe, Japan, and Australia. Now, invitations are coming from other parts of the world—rich and poor countries alike. Because of the complexities involved in making and administering the drug, only hospitals certified by Novartis can offer it.
"I have been traveling far more than my wife would approve," Grupp said recently by phone from Dubai in the United Arab Emirates. "We (CHOP and Penn) get all these emails from parents across the world. We're trying to look at access. We're really interested in figuring out how to help patients who won't have access in their own countries, at least not for years."
©2018 The Philadelphia Inquirer
Distributed by Tribune Content Agency, LLC.