Study shows NIH spent >$100 billion on basic science for new medicines
Federally funded research contributed to the science underlying all new medicines approved by the FDA over the past six years, according to a new study by Bentley University.
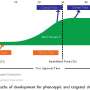
The new report from the Center for Integration of Science and Industry at Bentley University, published in the Proceedings of the National Academy of Sciences, shows that the United States government invested more than $100 billion in the basic research that led to new medicines approved by the Food and Drug Administration (FDA) between 2010 and 2016. Every one of the 210 new medicines approved over this six year period was associated with research funded by the National Institutes of Health (NIH). As much as $64 billion of this funding was associated with 84 innovative, first-in-class drugs, which treat disease through novel biological mechanisms or targets.
The article, titled "Contribution of NIH funding to new drug approvals, 2010-2016" is the first to measure the scale of the public sector contribution to new drug discovery and development, specifically the role of NIH funding for basic biomedical research.
"While basic research can sometimes seem esoteric, our analysis shows that a substantial fraction of NIH funded, basic research can be directly linked to the medicines first approved in this decade." said Dr. Fred Ledley, Director of the Center for Integration of Science and Industry, and the senior author on this study. "This data underscores the critical impact of government funding for basic biomedical research on the drug discovery and development process. Any reduction in this funding would inevitably slow the pipeline of new treatments for diseases that the public so desperately needs."
The Bentley study involved analysis of more than 2 million published research reports that were directly related to the 210 new medicines approved from 2010-2016 or to their biological targets. Of these, 600,000 published reports were identified as the work product of NIH-funded research projects. These projects involved more than 200,000 fiscal years of research funding and more than $100 billion in total cost. More than 90% of this funding was directly associated with research on the biological targets for drug action, rather than the drugs themselves, and represents basic biomedical research.
The contribution of NIH funding to new drug approvals is difficult to measure. Part of the difficulty is that much of the NIH research budget is dedicated to basic research, which is directed at exploring fundamental biological processes without specific applications or products in mind. The discovery of novel biological mechanisms and targets through basic research is an enabling step leading to the discovery and development of new medicines for untreatable diseases.
More information: Ekaterina Galkina Cleary el al., "Contribution of NIH funding to new drug approvals 2010–2016," PNAS (2018). www.pnas.org/cgi/doi/10.1073/pnas.1715368115