Scientists can now measure activity of key cancer cell-survival protein, an important step toward inhibiting it
A recent study from the University of Michigan Life Sciences Institute and the University of California, San Francisco, has opened new options to further develop a potential cancer-fighting therapy, clearing an early hurdle in the lengthy drug-discovery process.
The findings, published in the Journal of Biological Chemistry, reveal new ways to measure the activity of a protein that is associated with poor prognosis in cancer patients—heat shock protein 70, or Hsp70—and remove a barrier to developing potential Hsp70-based therapies.
When Hsp70 is present in increased levels, cancer cells are more likely to survive and become resistant to chemotherapeutics. Conversely, when this protein is inhibited in cells, tumor cells are less able to divide, and they eventually die.
Because of its apparent role in cancer cell survival, researchers are interested in developing drugs that block the protein's activity. But these efforts have been hindered by a lack of Hsp70 biomarkers—measureable surrogates that scientists can evaluate to ensure the compounds they are testing really do what they are supposed to do.
Scientists have found some small molecules that affect Hsp70 in an artificial environment, where they can directly measure Hsp70 activity. To move to the next steps in drug development, researchers need to know how the protein is responding in its natural environment.
"And it's a lot more difficult to measure Hsp70 inhibition in a cell," says lead study author Laura Cesa, Ph.D, a former U-M Program in Chemical Biology graduate student, now at the University of Copenhagen Biotech Research & Innovation Centre.
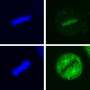
For this study, the researchers set out to determine whether Hsp70 has its own specific client proteins—other proteins that can serve as biomarkers for Hsp70.
"This was an interesting question from a basic research standpoint, but it also has exciting therapeutic indications," Cesa explains. "If we can show that inhibiting Hsp70 results in the degradation of its specific client proteins, then those could be used as biomarkers for future development of Hsp70 drugs for cancer therapy."
The team succeeded in identifying client proteins whose levels can be measured and are directly tied to the activity of Hsp70, meaning they can serve as a proxy for measuring Hsp70.
These biomarkers will now enable scientists to take the small molecules that inhibit Hsp70 in artificial environments and begin testing ways to develop them into actual cancer therapeutics.
The significance of these findings led the study to be chosen as the JBC Editors' Pick for the February 16 issue. This honor is reserved for the top 2 percent of the more than 6,600 papers published in the journal each year, in terms of the overall importance of the research.
More information: Laura C Cesa et al, X-Linked Inhibitor of Apoptosis Protein (XIAP) is a Client of Heat Shock Protein 70 (Hsp70) and a Biomarker of its Inhibition, Journal of Biological Chemistry (2017). DOI: 10.1074/jbc.RA117.000634