Investigational genome editing therapy in clinical trial for Hunter syndrome
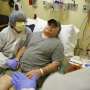
This week, a 40-year-old patient was treated at UNC's Clinical and Translational Research Center (CTRC) with SB-913, an investigational genome editing therapy for individuals with mucopolysaccharidosis type II (MPS II), a rare lysosomal storage disorder also known as Hunter syndrome.
This treatment marks a significant milestone for Joseph Muenzer, MD, Ph.D., and the CTRC. It was the first time that Muenzer, a pediatric biochemical geneticist who specializes in disorders such as Hunter syndrome, had the opportunity to administer a gene therapy treatment to a patient with this condition."I developed a mouse model for Hunter syndrome at UNC about twenty years ago to help develop therapies for this rare disorder," said Dr. Muenzer. "I spent many years working on gene therapy in my UNC research lab and it is with great pleasure that I am able to finally perform gene therapy on an individual with Hunter syndrome. Gene therapy has the potential to dramatically improve the quality of life for individuals with Hunter syndrome."
The treatment, SB-913, was developed by Sangamo Therapeutics, a biotechnology company in the San Francisco Bay Area in California. Sangamo is currently evaluating the treatment in a Phase 1/2 clinical trial, called the CHAMPIONS study. The patient at UNC was the third patient to receive the treatment so far as part of the CHAMPIONS study.
The CHAMPIONS study is an open-label clinical study designed to assess the safety, tolerability and preliminary efficacy of three dose levels of the SB-913 investigational genome editing therapy in up to nine adult males with Hunter syndrome. The study is sponsored by Sangamo.
"Dr. Muenzer and his team at UNC Chapel Hill are renowned experts in the treatment of MPS diseases and continue to lead the field into a new frontier of genomic medicine," said Ed Conner, MD, chief medical officer of Sangamo. "We are very pleased to work with them in this clinical trial evaluating SB-913."
Hunter syndrome is a rare progressive disorder that primarily affects males and is caused by mutations in the gene encoding the iduronate-2-sulfatase (IDS) enzyme. Individuals with Hunter syndrome can have a wide spectrum of clinical involvement from severe to attenuated, depending on the severity of the mutation and degree of residual enzyme activity. Children with the severe form of Hunter syndrome begin showing symptoms of developmental delay by age two or three and develop enlarged liver and spleen, airway and cardiac disease, skeletal abnormalities, hearing loss, short stature and if untreated die in their teenage years. Individuals with the attenuated form can have similar physical disease, but never develop cognitive impairment.
The therapy aims to treat the condition by using genome editing to insert a corrective gene into a precise location in the DNA of liver cells with the goal of enabling a patient's liver to produce a lifelong and stable supply of an enzyme that they lack.
Without the IDS enzyme, individuals with Hunter syndrome have progressive lysosomal accumulation of glycosaminoglycans resulting in cell and organ dysfunction and typically a shorten life-span. Approximately one in 100,000 to one in 170,000 individuals are born with the condition. Most individuals with MPS II receive weekly infusions of enzyme replacement therapy (ERT), the current standard-of-care treatment. Within a day of receiving ERT, however, IDS quickly returns to near undetectable levels in the blood.
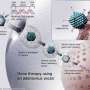
SB-913 is designed as a single treatment strategy intended to provide stable, continuous production of the IDS enzyme for the lifetime of the patient. SB-913 makes use of Sangamo's zinc finger nuclease (ZFN) genome editing technology to insert a corrective gene into a precise location in the DNA of liver cells.
To restrict editing to liver cells, the ZFNs and the corrective gene are delivered in a single intravenous infusion using AAV vectors that target the liver. The ZFNs enter the cells as inactive DNA instructions in a format designed only for liver cells to unlock. Once "unlocked," the ZFNs then identify, bind to and cut the DNA in a specific location within the albumin gene. Using the cells' natural DNA repair processes, liver cells can then insert the corrective gene for IDS at that precise location. The FDA has granted Orphan Drug, Fast Track and Rare Pediatric Disease designations to SB-913 for the treatment of MPS II.
The ability to permanently and precisely integrate the therapeutic IDS gene into the DNA differentiates Sangamo's in vivo genome editing approach from conventional AAV cDNA gene therapy and from lenti- or retroviral-based gene therapies that insert genes randomly into the genome.