Scientists say advanced vaccines could limit future outbreaks
Novel vaccine technologies are critical to improving the public health response to infectious disease threats that continually emerge and re-emerge, according to scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. In a perspective in The Journal of the American Medical Association, the experts highlight innovations that could significantly shorten the typical decades-long vaccine development timeline.
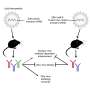
Historically, vaccines against viral diseases have used live-attenuated (weakened) viruses or inactivated whole viruses to induce protective immune responses. The development process often takes 15 to 20 years or more and requires virus cultivation, animal model testing, product formulation, immunogenicity testing and years of costly clinical trials. However, substantial technological advances of the past decade, such as synthetic vaccinology and platform manufacturing, can expedite the process and shorten manufacturing time, allowing clinical evaluation to begin sooner, according to the authors. Synthetic vaccinology uses information from viral gene sequencing to create DNA and mRNA molecules encoding viral proteins. Because this research does not require replicating "live" viruses, it does not need to be done in high-level containment facilities when developing vaccines for highly pathogenic viruses.
The perspective notes that once a vaccine platform is established, such as that for DNA or mRNA vaccines, potentially it can be applied to multiple pathogens, especially within virus classes or families. For example, NIAID's Vaccine Research Center quickly developed a candidate DNA vaccine for Zika virus with the same platform used previously for a related flavivirus, West Nile virus. Platform technologies enable scientists to apply a standardized manufacturing process to multiple vaccines and create a collective database on their safety as well, which can shorten the preclinical development period to as little as several months, according to the authors. The perspective concludes that modern vaccine technology and improved surveillance in developing countries ultimately can help us better prepare for emerging infectious disease threats.
More information: BS Graham et al. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. The Journal of the American Medical Association DOI: 10.1001/jama.2018.0345 (2018).