Large aggregates of ALS-causing protein might actually help brain cells
Scientists at the UNC School of Medicine have made a significant advance in the understanding of the complex and fatal neurodegenerative disease amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease.
Autopsy studies of ALS patients often reveal the accumulation of large, fibrous aggregates of a protein called SOD1 in disease-affected motor neurons. Researchers have hypothesized that these fibrils are what kill neurons and cause ALS in some people. But in a study published in the Proceedings of the National Academy of Sciences, scientists at the University of North Carolina at Chapel Hill found evidence that these large SOD1 fibrils protect rather than harm neurons.
"This is potentially an important finding not only for ALS research but for neurodegenerative disease research in general, because the formation of fibril aggregates is so common in these diseases," said senior author Nikolay Dokholyan, PhD, the Michael Hooker Distinguished Professor of Biochemistry and Biophysics at UNC-Chapel Hill.
Large, often fibril-type protein aggregates are in fact the most obvious pathological features of Alzheimer's disease, Parkinson's disease, Huntington's disease, ALS, and other major neurodegenerative diseases. Many of the candidate drugs developed in recent years were designed to clear these protein aggregates. But none of these fibril-targeting strategies have proven effective in large clinical trials. Laboratory studies also have largely failed to prove that large SOD1 fibrils are harmful to neurons.
At the same time, researchers have found that much smaller protein clusters called oligomers - made of only a few copies of these proteins - can be highly toxic to motor neuron-like cells grown in the lab and thus are more likely to be the chief causes of brain-cell death in these diseases.
In a 2016 study, for example, Dokholyan's lab found evidence that "trimer" structures made of just three copies of the SOD1 protein are toxic to the type of neuron affected in ALS.
For the new study, Dokholyan's team, including lead author Cheng Zhu, PhD, a postdoctoral researcher in his lab, conducted complicated experiments to compare how trimers affect neurons to how larger fibrils affect neurons.
"One challenge is that the smaller structures such as trimers tend to exist only transiently on the way to forming larger structures," Zhu said. "But we were able to find an SOD1 mutation that stabilizes the trimer structure and another mutation that promotes the creation of the larger fibrils at the expense of smaller structures. So, we were able to separate the effects of these two species of the protein."
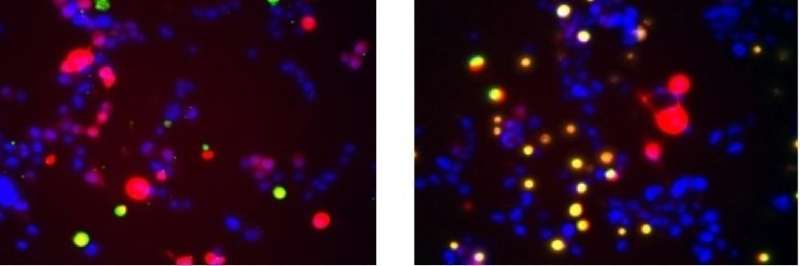
The researchers expressed the mutant SOD1 proteins in test cells that closely resemble the muscle-controlling neurons killed in ALS. They found - as they did in the 2016 study - that when these cells expressed SOD1 mutants that predominantly form trimers, the cells died much more quickly than control cells containing normal SOD1. The trimer-expressing cells even died more quickly than cells expressing mutant forms of SOD1 that are found in severe hereditary ALS cases.
"Looking at various SOD1 mutants, we observed that the degree of toxicity correlated with the extent of trimer formation," Zhu said.
On the other hand, the viability of cells containing mutant SOD1 that strongly forms fibrils but suppresses trimers tended to be similar as wild-type SOD1, suggesting that the fibrils are protective, not merely less toxic.
This suggests SOD1 fibrils aren't the problem in SOD1-linked ALS; they might be a solution. "Taking a drug to promote fibril formation could be one way to reduce toxicity in SOD1-ALS," Dokholyan said.
An alternative strategy, he noted, would be to limit the formation of trimers or other small, toxic SOD1 oligomers. SOD1 normally works in cells as a two-copy structure, a dimer. Trimers and other abnormal structures appear to originate when the dimers fall apart. So Dokholyan and colleagues are looking for potential drug molecules that can stabilize the dimers.
SOD1 is linked to a significant proportion of ALS cases. Mutations in the SOD1 gene account for about 12 percent of ALS cases that run in families. All of these mutations destabilize the protein's normal structure and promote abnormal SOD1 structures. SOD1 mutations also appear to account for about 1.5 percent of cases that do not obviously run in families.
"Although SOD1-associated ALS represents a small fraction of all ALS cases, uncovering the origins of neurotoxicity in SOD1 aggregation may shed light on the underlying causes of an entire class of neurodegenerative diseases," Dokholyan said.The next steps for Dokholyan's lab is to pinpoint downstream cellular mechanisms of toxicity of pathological trimeric SOD1 and find drugs that mitigate the formation of trimers.
More information: Cheng Zhu el al., "Large SOD1 aggregates, unlike trimeric SOD1, do not impact cell viability in a model of amyotrophic lateral sclerosis," PNAS (2018). www.pnas.org/cgi/doi/10.1073/pnas.1800187115