Abdominal fat secretes novel adipokine promoting insulin resistance and inflammation
An international research team in which the DZD is participating has identified a novel adipokine that favors the development of insulin resistance and systemic inflammation. In cases of severe obesity, this adipokine is secreted by the adipocytes of the abdominal fat tissue and released into the bloodstream. The new findings could contribute to the development of alternative approaches for the treatment of diseases caused by obesity. The researchers have now published their results in the journal Diabetologia (Hörbelt et al, 2018) of the European Association for the Study of Diabetes (EASD).
More than 2.8 million people die each year due to conditions related to overweight and obesity. Overweight and the associated metabolic syndrome increase the risk of type 2 diabetes, specific types of cancer and cardiovascular disease. Scientific findings in recent years have confirmed this increased risk. The cause of the sequelae are chronic inflammatory responses. However, the molecular mechanisms that lead to these overweight-related inflammatory processes are still largely unknown. This is the starting point for the study of the international team of scientists led by PD Dr. Natalia Rudovich (Spital Bülach; Charité - Universitätsmedizin Berlin), Prof. Dr. Margriet Ouwens (German Diabetes Center Düsseldorf) and PD Dr. Olga Pivovarova of the German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE).
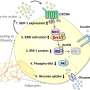
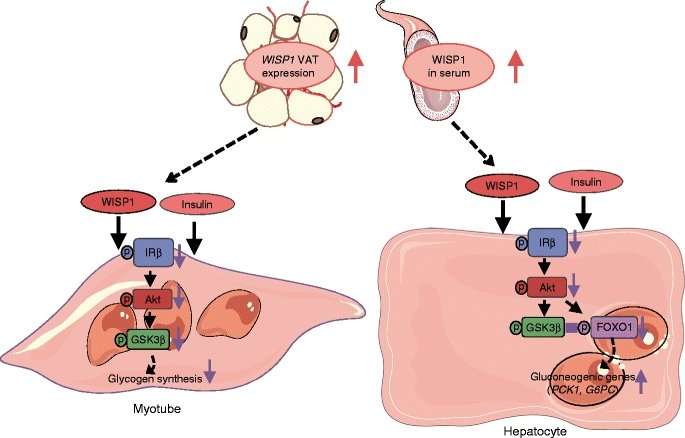
The researchers showed for the first time how the protein molecule Wingless-type signaling pathway protein-1 (WISP1) directly impairs insulin action in muscle cells and in the liver and thus leads to reduced insulin sensitivity. Already in 2015, the team led by the physician Rudovich and the biologist Pivovarova identified WISP1 as another possible link between obesity and systemic inflammatory responses. WISP1 was previously associated with the regulation of bone growth, the development of certain types of cancers and pulmonary fibrosis.
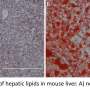
The current study shows that WISP1 cancels insulin-induced inhibition of glucose production (gluconeogesis) in murine hepatocytes and glycogen synthesis in human muscle cells. The synthesis quantity of the WISP1 protein correlates with the blood glucose levels in the oral glucose tolerance test (OGTT) and with the circulating level of heme oxygenase-1 (HO-1), an enzyme that promotes systemic inflammation, especially in obesity (7). "We suspect that increased WISP1 production from abdominal fat could be one of the reasons why overweight people often have an impaired glucose metabolism," said first author Tina Hörbelt of the German Diabetes Center Düsseldorf, a partner of the DZD."One possible cause of increased WISP1 production and secretion from the abdominal fat cells could be the poor oxygen supply (hypoxia) of the tissues. This could lead to systemic inflammatory responses," explained DIfE researcher Pivovarova.
The new findings open up alternative approaches to the treatment of diseases caused by obesity. "For example, novel drugs could target and specifically prevent the WISP1 effect on muscles and liver cells and thus lead to improved insulin action in these tissues," said Rudovich, head diabetologist and endocrinologist at Spital Bülach. "However, it is still a long way from basic research (8) to a viable treatment", the physician added. Nevertheless, the new findings would already contribute to a better understanding of the relationships between obesity, the immune system and metabolic diseases.
More information: Tina Hörbelt et al, The novel adipokine WISP1 associates with insulin resistance and impairs insulin action in human myotubes and mouse hepatocytes, Diabetologia (2018). DOI: 10.1007/s00125-018-4636-9