Homogeneous BTK occupancy assay
In a new SLAS Discovery article, Helen Yu and a team of researchers at Gilead Sciences, Inc. (Foster City, CA) present a time-resolved fluorescence resonance energy transfer-based Bruton's tyrosine kinase (BTK) occupancy assay that can measure target engagement in peripheral blood mononuclear cells (PBMCs) and in lymph-node and bone-marrow samples. The assay provides accurate, quantitative assessment of BTK occupancy and currently is in use in ongoing tirabrutinib clinical studies.
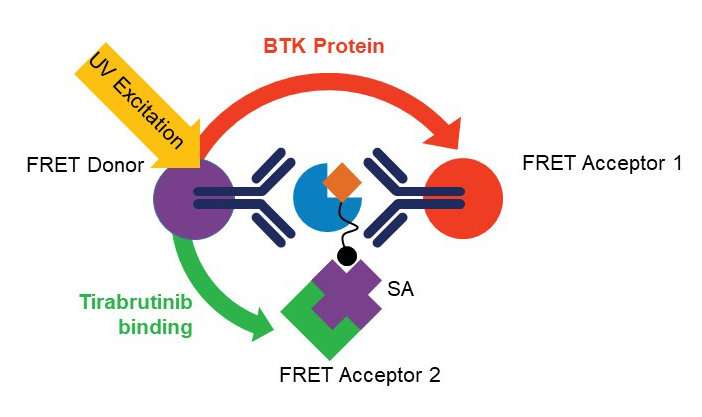
The homogenous BTK occupancy assay's multiplexed format allows simultaneous measurement of tirabrutinib bound and total BTK levels, reducing clinical sample requirements and avoiding sampling variability observed with traditional single analyte assays.
Yu et al. take advantage of the dual wavelength emission of terbium conjugated anti-BTK antibody to serve as the energy donor for two fluorescent energy acceptors with distinct excitation and emission spectra: G2-streptavidin-bound biotinylated tirabrutinib (detects free BTK) and D2-coupled second anti-BTK antibody that binds to a different BTK epitope (detects total BTK). Additionally, the use of a common fluorescence donor serves to normalize the detection of total and free BTK with respect to each other. The assay is characterized and quantified using full-length purified recombinant human BTK protein and PBMCs derived from healthy volunteers and patients with CLL. The authors demonstrate the assay's utility using cells derived from samples from patients with CLL and DLBCL.
BTK is expressed in B cells and myeloid cells and plays an essential role in the B-cell receptor (BCR) signaling pathway. Tirabrutinib (GS-4059/ONO-4059) is a second-generation, potent, selective, irreversible BTK inhibitor. It is currently in clinical development as a treatment for chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), and other lymphoid malignancies.
Integrated drug discovery and clinical development programs increasingly emphasize target engagement as a translational approach to inform pharmacodynamic and efficacy assessments. Correlation of tirabrutinib target engagement at the molecular level with pharmacological and phenotypic disease observations is crucial for establishing the appropriate clinical doses.
More information: Helen Yu et al, Homogeneous BTK Occupancy Assay for Pharmacodynamic Assessment of Tirabrutinib (GS-4059/ONO-4059) Target Engagement, SLAS DISCOVERY: Advancing Life Sciences R&D (2018). DOI: 10.1177/2472555218786165