How lifestyle affects our genes

In the past decade, knowledge of how lifestyle affects our genes, a research field called epigenetics, has grown exponentially. Researchers at Lund University have summarised the state of scientific knowledge within epigenetics linked to obesity and type 2 diabetes in a review article published in the scientific journal Cell Metabolism.
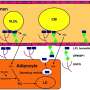
Epigenetic mechanisms control the activity of different genes. Disruptions in the epigenetic machinery may lead to diseases such as obesity and type 2 diabetes. This review summarises the role of epigenetic changes in different human tissues of relevance for metabolism—for example, in adipose tissue, skeletal muscle, pancreatic islets (which, among other things, contain insulin-producing cells), liver and blood—linked to obesity and type 2 diabetes.
"Epigenetics is still a relatively new research field; however, we now know that epigenetic mechanisms play an important role in disease development. Similarly, epigenetic patterns are affected by disease. Variations in genetic material (DNA), age, exercise and diet also have an impact on epigenetic variation," says Charlotte Ling, professor and pioneer in the field of epigenetics and diabetes who authored the review together with researcher Tina Rönn. The researchers detail the latest findings in epigenetics in the following categories:
The significance of diet
Research shows that our diet affects the epigenome in several tissues that are important for metabolism. The authors of the article write that several intervention studies have been performed where participants were divided into groups with different diets to study the impact on the epigenome. Some examples:
- A high-fat diet for five days led to changes in both the gene activity and DNA methylation patterns in skeletal muscle and adipose tissue.
- Saturated or polyunsaturated? In one study, the participants ate muffins made with either saturated or polyunsaturated fat for seven weeks. All the participants gained the same amount of weight; however, those who ate a diet high in saturated fat were affected to a greater extent by visceral fat accumulation and stored the fat in their livers. DNA methylation in the adipose tissue was affected differently between the two groups.
"Both visceral obesity and fat accumulation, for example, in the muscles and liver, are risk factors for diabetes and cardiovascular disease. Another important observation was that it seemed easier to induce methylation changes by eating a high-fat diet than to reverse them at a later stage with a control diet," says Charlotte Ling.
Physical activity
It is well known that regular exercise protects and prevents individuals at high risk for type 2 diabetes from developing the disease. Exercise has a beneficial effect not only on glucose homeostasis and whole-body energy balance but also on the immune system.
The authors of the article describe several studies that have shown that exercise has an impact on DNA methylation (see fact box) and on the function of genes in skeletal muscle and adipose tissue.
- A study from 2013 shows that epigenetic changes that occur during exercise have an impact on the metabolism of fatty acids in adipose tissue by increasing the activity in specific genes.
- Exercise for short or long periods of time have a clear impact on DNA methylation and is different between both genes and tissues.
"Epigenetics can explain why different people respond differently to exercise," says Tina Rönn.
Aging
Aging is associated with increased abdominal obesity, insulin resistance and type 2 diabetes. A better understanding of age-related mechanisms that lead to obesity and type 2 diabetes can therefore lead to new and preventive treatment methods.
The research shows that:
- The process of aging is characterised by significant variation in the epigenome where the genes lose or gain new methylations.
- Obesity seems to have an impact on the age-driven epigenetic changes, which provides a molecular link between aging and obesity.
"Despite DNA methylation being a stable epigenetic marker, we know that the epigenome changes over time," says Charlotte Ling.
The significance of genes
Our genes are regulated by an extremely complex process that starts in the molecular building blocks of genes, the so-called nucleotides, which make up a strand of DNA. Then add DNA methylation, RNA molecules and histone modifications (chemical groups that attach to and impact the proteins around which our long DNA spiral is wound), that are all also affected by both genetic and environmental factors.
Studies show that:
- Since DNA methylation mainly occurs in specific places in our DNA, hereditary variations in the composition of genes can impact the possibility for methyl groups to attach to the genes.
- A study on pancreatic islets shows that half of all gene variants that can be linked to type 2 diabetes also create or remove sites where DNA methylation can take place, and of these, some could also be linked to the function of the genes and the ability of the cells to secrete insulin.
- Different genetic variants mediate the effect on metabolic traits including BMI (Body Mass Index) and HbA1c via altered levels of DNA methylation.
Is the epigenome heritable?
"It is an interesting and challenging hypothesis that epigenetic inheritance contributes to evolution," write the authors. The epigenome is variable and adapts faster to the changes in our lifestyle over recent decades than our genes can through mutations. During the embryonic stage, the most active epigenetic re-programming takes place. The authors of the article describe that what a woman is exposed to during pregnancy not only affects her and her unborn child, but also her grandchildren due to the effect on the embryo's own reproductive cells. Both eggs and what will later become sperms are already formed during the embryonic stage and constitute parts of the genetic material within the reproductive cells.
"To be able to claim that epigenetic changes can be inherited across several generations, you have to be able to prove changes also in the fourth generation and, to our knowledge, there is only limited, if any, evidence of transgenerational epigenetic inheritance in humans."
There are now an increasing number of studies on the transfer of epigenetic information from the father. For example, it has been shown that sperms also have an epigenome that is affected by environmental factors and which is likely to have an effect on the next generation:
- A study from 2016 shows that the sperm DNA methylome from obese men who have had bariatric surgery was remodelled after the operation.
- An earlier study also showed that exercise has the ability to reprogram the sperm methylome, but how these changes are inherited and beneficial for the offspring remains to be established.
How can epigenetics contribute to novel treatments?
It is already known that regular exercise and a healthy diet can prevent individuals at risk for type 2 diabetes from developing the disease. It is therefore important to be able to identify these individuals at an early stage.
- "In the future, it may be possible to use epigenetic biomarkers to predict who is at high risk of developing obesity and type 2 diabetes, provided that they are easily accessible, for example in the blood," write the authors of the article.
- New technical methods that make it possible to direct drugs at a specific target provide the opportunity to develop new treatment methods. These could be aimed at epigenetic modifications as these can be altered.
There are already drugs that have an effect on DNA methylation and histone modification. These have been tested with good results, e.g. for leukaemia. The authors say epigenetic drugs could also have an effect on obesity and type 2 diabetes as studies have shown in several cases that they improve insulin secretion.
"Given that type 2 diabetes is a chronic disease, it would need to be a lifelong drug, and therefore advantages need to be weighed against disadvantages and any side effects must be studied first," says Charlotte Ling.
Facts/Epigenetics:
Our genetic material, the DNA strand, contains all genes and is found in the body's cells. We inherit the DNA and it cannot be changed. DNA methylation is the most studied epigenetic marker (and the part this review focuses on) and involves a chemical compound—methyl groups—attached to genes and regulatory regions of the DNA, thereby affecting which genes are turned on and off.
When the first studies of epigenetics and type 2 diabetes were completed just over ten years ago, DNA methylation of chosen candidate genes or small parts of the genome were analysed. With technical advances it became possible to analyse all the genes in the genome in the smallest components where the methylations take place, the so-called CpG sites. Currently, it is possible to study the whole epigenome with a technique called whole genome bisulfite sequencing (WGBS), where over 80 percent of all CpG sites in the whole genome are analysed. Aside from DNA methylation, the epigenome also includes different histone modifications and small non-coding RNAs.
More information: Charlotte Ling et al, Epigenetics in Human Obesity and Type 2 Diabetes, Cell Metabolism (2019). DOI: 10.1016/j.cmet.2019.03.009