New blood test on horizon for the 1 in 10 children who suffer common liver disease
A new blood test could become clinical practice within five years, reducing the need for a liver biopsy in the management of paediatric Non-Alcoholic Fatty Liver Disease (NAFLD), as a major new international paediatric liver registry collaboration yields early results.
Researchers are today presenting findings, at the 52nd Annual Meeting of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition, which indicate that blood tests could replace the need for a liver biopsy in children suffering with NAFLD. Currently, if a doctor is concerned a child has scarring or inflammation of the liver they may recommend a liver biopsy. The study looked at 67 children with NAFLD and found that different types of fats in the blood were associated with features of fatty liver on liver biopsy, allowing researchers to determine the presence of inflammation and scarring—also known as non-alcoholic steatohepatitis and fibrosis.
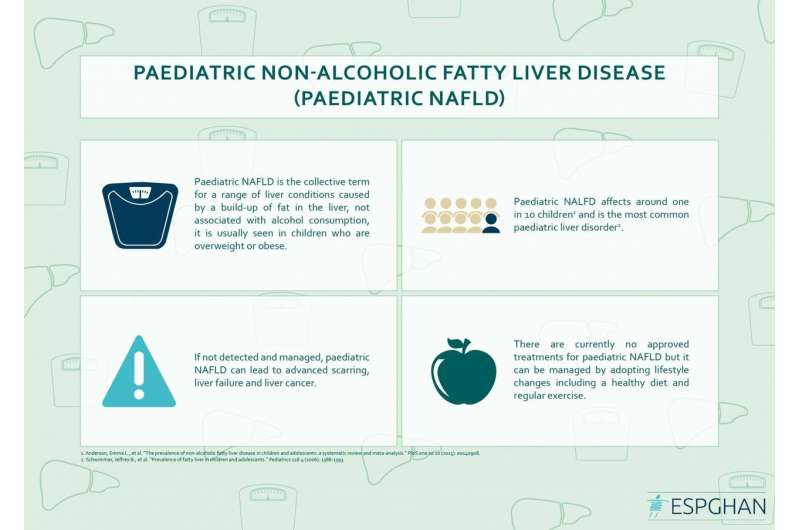
Non-Alcoholic Fatty Liver Disease affects around one in 10 children and is the most common paediatric liver disorder. It can progress to advanced scarring (cirrhosis), liver failure, and liver cancer. Despite this, the natural history of the condition is poorly understood and there are currently no approved treatments or drugs in clinical trials for children. In response to this, researchers from across Europe have come together to pool resources in order to better understand the condition and how to treat it.
Liver biopsy is currently the most accurate test for NAFLD and the only method routinely used in practice for assessing the presence of scarring or inflammation. However, biopsy is invasive, resource intensive, costly, prone to sampling error and carries a small risk of significant complications. Therefore, the availability of an accurate and non-invasive marker to replace the need for liver biopsy, both in routine practice and in a clinical trial setting, is a major breakthrough for children, parents and healthcare professionals.
To date, all European drug trials and assessments of non-invasive markers in paediatric NAFLD have been single-centre studies, which limits the generalisability of findings. This is particularly pertinent given the variation in clinical practice at different centres.
This research is the first major finding to be reported from the European Paediatric Non-Alcoholic Fatty Liver Disease Registry (EU-PNALFD registry), an international collaboration of 11 specialist and non-specialist centres in six European countries, led by Dr. Jake Mann. When fully operational, the registry will have enrolled up to 2,000 children, including 500 with biopsy-proven paediatric NAFLD, and follow-up will continue for up to 30 years.
Commenting on the findings, registry co-ordinator Dr. Jake Mann said:
"It is early days but the results of the research are promising and could help shift the way we understand and manage paediatric NAFLD: saving resources, time, and stress for children and their parents. The new multi-centre registry provides us with an opportunity to tackle these challenges. The EU-PNAFLD registry will facilitate recruitment into interventional clinical trials as well as imaging, biomarker, and translational studies, plus allow greater understanding of the long-term natural history of NAFLD. The ultimate aim is to understand the condition sufficiently to intervene and slow disease progression so we can reduce the number of patients requiring liver transplantation later in life."