Long-term dupilumab benefits adolescents with eczema
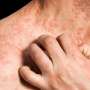
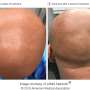
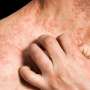
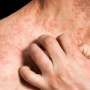
Results from a phase IIa open-label trial and a subsequent phase III open-label extension trial reinforce findings from an earlier short-term trial that adolescents with moderate-to-severe atopic dermatitis, or eczema, can experience significant improvements with dupilumab. The results from these latest studies, which are reported in the British Journal of Dermatology, demonstrate the long-term safety and efficacy of the medication for up to 52 weeks of treatment.
Dupilumab works by stopping the action of certain substances in the body that cause the symptoms of eczema. Specifically, it is a monoclonal antibody against interleukin-4 receptor alpha.
"Adolescents with moderate-to-severe atopic dermatitis have a high disease burden that negatively affects quality of life, and patients are in need of therapies that can be used long-term," said senior author Ashish Bansal, MD, of Regeneron Pharmaceuticals, Inc., in Tarrytown, New York. "Results from these trials show that dupilumab provides substantial and sustained clinical benefit to these patients with an acceptable safety profile."
More information: M.J. Cork et al, Dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: results from a phase II a open‐label trial and subsequent phase III open‐label extension, British Journal of Dermatology (2019). DOI: 10.1111/bjd.18476