New treatment discovered for rare eye disease may prevent blindness
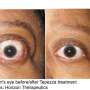
Patients with thyroid eye disease who used the minimally invasive insulin-like growth factor I blocking antibody, teprotumumab, experienced improvement in their symptoms, appearance and quality of life, according to a study recently published in the New England Journal of Medicine.
The randomized, double-blind, placebo-controlled trial was conducted by the department of surgery at Cedars-Sinai and at other medical centers nationwide.
Thyroid eye disease is a rare and vision-threatening autoimmune condition that causes the muscles and fatty tissues behind the eye to become inflamed and enlarged, leading to bulging of the eyes. In addition to the bulging appearance, patients can experience double vision and light sensitivity. The disease can lead to blindness.
"The study demonstrates that medical treatment with teprotumumab is effective at reversing the manifestations of disease, providing new hope for patients," said the study's principal investigator Raymond Douglas, MD, Ph.D., director of the Orbital and Thyroid Eye Disease Program at Cedars-Sinai.
Patients received the drug intravenously once every week for three weeks over a 21-week period. Results showed:
- Patients who were administered teprotumumab experienced effective response in two doses or six weeks of administration.
- After 24 weeks, the study showed 83% of people on the drug had measurable reduction in eye bulge versus 10% of those on a placebo.
- The overall response rate was 78% among those taking the drug compared to 7% of people taking a placebo.
The new discovery contributed to the fast track drug approval by the U.S. Food and Drug Administration, marketed under the brand name Tepezza, making it the first drug approved for the condition.
"Other than highly invasive surgical procedures, patients with thyroid eye disease had no real treatment alternatives," Douglas said. "This is a medical breakthrough for a very large percentage of the patient population to receive an alternative medical infusion treatment with great results, quickly."
Teprotumumab is a fully human monoclonal antibody which blocks the inflammatory autoimmune pathophysiology that underlies thyroid eye disease.
"This treatment has the potential to alter the course of the disease, potentially sparing patients from needing multiple invasive surgeries by providing an alternative, nonsurgical treatment option," said Wiley Chambers, M.D., deputy director of the Division of Transplant and Ophthalmology Products in the FDA's Center for Drug Evaluation and Research.