New targeted agent produces considerable responses in patients with uterine cancer
In its first clinical trial in patients with a hard-to-treat form of uterine cancer, a targeted drug that subjects tumor cells to staggering levels of DNA damage caused tumors to shrink in nearly one-third of patients, investigators at Dana-Farber Cancer Institute report.
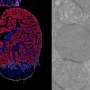
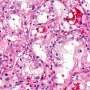
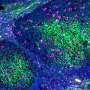
The preliminary results, to be presented online at Thursday's virtual session of the Society for Gynecologic Oncology (SGO) Annual Meeting on Women's Cancer, demonstrated strong activity of WEE1-directed therapy in uterine serous carcinoma (USC), which accounts for about 10% of uterine cancers but up to 40% of deaths from the disease, trial leaders say.
The drug tested in the study—adavosertib—takes advantage of an inherent weakness in the relentless growth of some cancer cells. Their non-stop proliferation creates a condition known as replication stress, where their ability to duplicate their DNA effectively is significantly impaired. The cell cycle—the carefully choreographed process by which cells grow, copy their DNA, and divide into two daughter cells—includes several checkpoints that halt the cycle so DNA can be inspected and repaired, if necessary. In some cancers, a checkpoint fails to function due to a genetic mutation or other problem, allowing the cycle to proceed even as DNA damage accumulates.
USC is one such cancer. More than 90% of cases are marked by a mutation or other abnormality in the TP53 gene, which plays a critical role in the checkpoint between the first phase of cell growth and the DNA-duplication phase. Without a working TP53 gene, cells can barrel into the DNA-duplication phase with extensive DNA damage on board.
The absence of functional TP53 places enormous strain on a checkpoint further on in the cell cycle called G2/M. Providing a final quality check, G2/M, guards the entry to mitosis, the act of dividing into two daughter cells. Hobbling G2/M by blocking one of the proteins involved in it could burden tumor cells with so much DNA damage that they cannot survive.
That is the strategy behind adavosertib, which targets a protein called WEE1 that helps regulate the G2/M checkpoint. The new trial marked the first time the drug, which has been tested in patients with other cancers, including breast and ovarian cancer, was tested in patients with USC.
The trial involved 35 patients, all of whom had previously been treated with platinum-based chemotherapy. They took adavosertib orally on a set schedule. At a median follow-up of 3.8 months, 10 of 34 patients who could be evaluated, had shrinkage of their tumors—a response rate of almost 30%.
In some cases, the responses were exceptionally durable, with some patients still responding more than a year after undergoing treatment, study leaders say.
The most common adverse side effects of the treatment were anemia, diarrhea, nausea, and fatigue.
"Adavosertib demonstrated remarkable activity as a single agent in this group of patients," says the study's lead author, Joyce Liu, MD, MPH, of Dana-Farber. "It's especially encouraging in a disease such as USC, for which current treatments are of limited effectiveness."