Discovering the secrets of the enigmatic caspase-6

St. Jude Children's Research Hospital scientists have identified previously unknown functions of the enigmatic enzyme caspase-6. The findings show that caspase-6 is a key regulator of innate immunity, inflammasome activation and host defense. Modulation of caspase-6 could be beneficial for treating viral diseases like influenza and other inflammatory diseases including cancer. The work appears as an advance online publication today in Cell.
Caspases are a family of enzymes that regulate programmed cell death (how a cell self-destructs), inflammation and other biological functions. Caspase-6 has previously been characterized as an executioner caspase in a non-inflammatory form of cell death called apoptosis. Caspase-6 has also been linked to neurological disorders like Alzheimer's disease and Huntington disease. However, the full range of the enzyme's function was not well understood. Now, researchers have discovered for the first time how caspase-6 regulates the ZBP1-NLRP3 inflammasome.
"We contributed to the fundamental understanding of caspase-6, which has remained a mystery in the field for decades," said senior author Thirumala-Devi Kanneganti, Ph.D., of the St. Jude Department of Immunology. "Caspase-6 has essential functions in innate immunity, inflammation and in driving PANoptosis."
Cell death and innate immune function
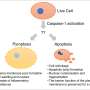
The Kanneganti laboratory previously was first to identify ZNA-binding protein 1 (ZBP1) as an innate immune sensor of influenza, an RNA virus. Their work also revealed that ZBP1 triggers inflammatory cell death in the form of pyroptosis, apoptosis and necroptosis, which together are known as PANoptosis.
PANoptosis is an inflammatory death pathway regulated by components of a structure termed the PANoptosome, which mediates cell death that cannot be assigned to any of the single cell death pathways described previously. In this study, the scientists found that caspase-6 played a critical role in this process.
The researchers found that caspase-6 interacts with RIPK3 to facilitate the recruitment of RIPK3 to the ZBP1-PANoptosome. This makes caspase-6 crucial for assembly of this ZBP1-mediated inflammatory cell death-inducing complex. In line with these findings, the researchers demonstrated that caspase-6 is required for ZBP1-mediated PANoptosis during viral infection.
"Caspase-6 deficiency in mice leads to increased susceptibility to influenza virus infection and higher levels of viral replication in the lungs," said first author Min Zheng, Ph.D., of the St. Jude Department of Immunology. "It is likely that the caspase-6-mediated inflammatory cell death pathway is essential to fighting other viruses that activate similar innate immune pathways, potentially including other respiratory viruses."
The discovery that caspase-6 is a key component in cell death processes has diverse implications for human health, suggesting that modulation of caspase-6 could be a beneficial approach to infectious and inflammatory disease treatment.
The other authors are Rajendra Karki and Peter Vogel, both of St. Jude.
More information: Min Zheng et al, Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense, Cell (2020). DOI: 10.1016/j.cell.2020.03.040